February 2023 Medical Image of the Month: Reversed Halo Sign in the Setting of a Neutropenic Patient with Angioinvasive Pulmonary Zygomycosis
 Thursday, February 2, 2023 at 8:00AM
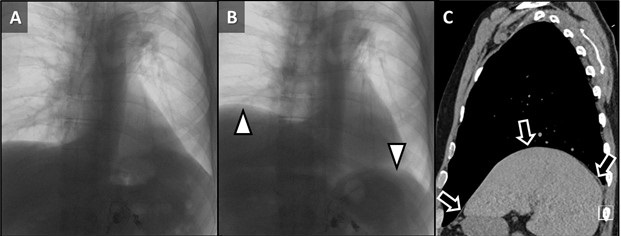
Thursday, February 2, 2023 at 8:00AM 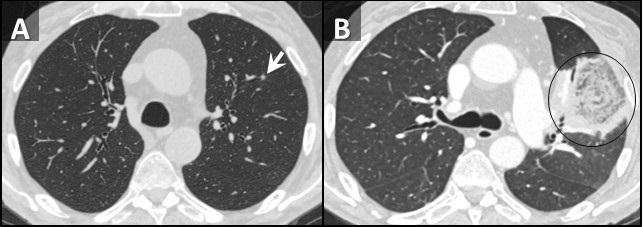 Figure 1. Axial reconstructions from unenhanced (A) and enhanced (B) chest CTs performed 1 week prior to admission (A) and at admission (B) demonstrating rapid interval increase in size of an initially small left upper lobe nodule (arrow) with extensive central necrosis manifesting as a “reversed halo” sign (circled, B).
Figure 1. Axial reconstructions from unenhanced (A) and enhanced (B) chest CTs performed 1 week prior to admission (A) and at admission (B) demonstrating rapid interval increase in size of an initially small left upper lobe nodule (arrow) with extensive central necrosis manifesting as a “reversed halo” sign (circled, B).
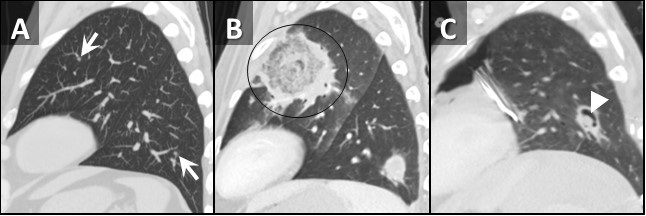 Figure 2. Sagittal reconstructions from unenhanced (A, C) and enhanced (B) chest CTs through the left lung performed 1 week prior to admission (A), at admission (B), and 2 weeks after admission (C). Small nodules on initial CT (arrows, A) rapidly grow with prominent central necrosis (circle, B). The follow up CT after the patient started improving demonstrates an “air crescent” sign (arrowhead, C) consistent with improving angioinvasive fungal infection.
Figure 2. Sagittal reconstructions from unenhanced (A, C) and enhanced (B) chest CTs through the left lung performed 1 week prior to admission (A), at admission (B), and 2 weeks after admission (C). Small nodules on initial CT (arrows, A) rapidly grow with prominent central necrosis (circle, B). The follow up CT after the patient started improving demonstrates an “air crescent” sign (arrowhead, C) consistent with improving angioinvasive fungal infection.
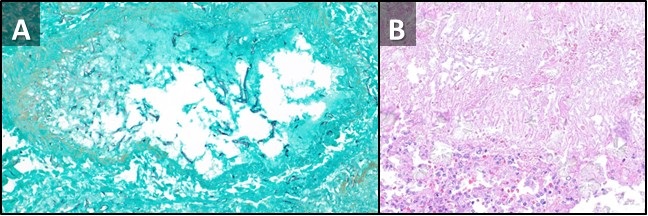 Figure 3. Low power view, GMS special stain (A) demonstrating a pulmonary artery with fungal elements invading into the wall and out into the surrounding lung parenchyma. There are variable and broad hyphae, with rare septation, many short fragments compatible with Rhizopus species grown in fungal culture. Low power view, H & E stain (B) from a different portion of the sample demonstrating fungal hyphae and spores with thinner morphology, right angle-branching, and calcium oxalate crystals, morphologically compatible with Aspergillus. This may represent secondary colonization of damaged lung.
Figure 3. Low power view, GMS special stain (A) demonstrating a pulmonary artery with fungal elements invading into the wall and out into the surrounding lung parenchyma. There are variable and broad hyphae, with rare septation, many short fragments compatible with Rhizopus species grown in fungal culture. Low power view, H & E stain (B) from a different portion of the sample demonstrating fungal hyphae and spores with thinner morphology, right angle-branching, and calcium oxalate crystals, morphologically compatible with Aspergillus. This may represent secondary colonization of damaged lung.
A 66-year-old man presented to our emergency department with fever and lethargy. A CBC demonstrated profound neutropenia with an absolute neutrophil count of <0.50x109 cells/L (critically low). The patient was admitted and workup for febrile neutropenia was begun. The patient’s past medical history includes CLL (recently confirmed to be in remission by bone marrow biopsy), hypogammaglobulinemia/capillary leak syndrome (presumably related to obinutuzumab therapy, for which patient receives monthly IVIG), and coccidioidomycosis (for which the patient has been followed by infectious disease at our institution, is on fluconazole). An outpatient chest CT performed 1 week prior to presentation to follow up pulmonary nodules demonstrated a few scattered small, but new, inflammatory-appearing nodules (Figure 1A, 2A).
A repeat chest CT was performed at time of admission, 7 days after the initial CT, which demonstrated marked interval increase in size of the small nodules, now represented as large areas of mass-like consolidation including a large finding in the left upper lobe displaying a reversed-halo sign (Figure 1B, 2B). Rapidly progressive fungal infection in the setting of neutropenia was favored. Due to rapid clinical deterioration and development of sites of infection outside the lungs, the decision was made to resect the left upper lobe for source control. The patient tolerated the procedure well, pathology from the specimen demonstrated pulmonary angioinvasive zygomycosis (mucormycosis) with broad areas of hemorrhagic pulmonary infarction, neutrophilic infiltrates and organizing hemorrhagic pneumonia. There were many invasive fungal organisms extending through the infarcted lung tissue. A culture of the lung showed Rhizopus species. There was prominent fungal angioinvasion with thrombosis in and around the infarcted lung. There were additional fungi in a bronchus that were thinner with more spores, septations, and elaborating oxalate crystals that were more consistent with Aspergillus species suggesting polymicrobial fungal infection. The patient was started on amphotericin B and posaconazole as well as filmgastrin. His neutropenia slowly improved, as did his clinical situation. A follow-up CT performed 2 weeks later demonstrated an air-crescent sign in the left lower lobe consistent with improving angioinvasive fungal infection in the setting of resolving neutropenia (Figure 2C).
The reversed halo sign consists of a finding of peripheral consolidation and central ground glass, in counter distinction to the CT halo sign, which consists of a nodule or mass (or mass-like consolidation) surrounded by ground glass (1). Interestingly, the halo sign was initially described in the setting of angioinvasive aspergillus infection (2), yet the opposite “reversed halo” sign is, in this case and many other cases, also described in the setting of invasive pulmonary fungal infection (3). The reversed halo sign was classically described in the setting of cryptogenic organizing pneumonia (4), where there is central disease clearing. This sign is also described as the “atoll” sign (5), representing relatively normal, improving lung in that situation. In the setting of invasive fungal infection, the central ground glass represents the opposite situation: dead, necrotic lung rather than improving lung. Although organizing pneumonia and invasive fungal infection are well-recognized causes of the reversed halo sign, the sign is by no means specific. Reversed halo signs can be seen in a wide variety of pathologies including paracoccidioidomycosis, pneumocystis pneumonia, tuberculosis, community-acquired pneumonia, lymphomatoid granulomatosis, granulomatosis with polyangiitis, lipoid pneumonia, sarcoidosis, pulmonary infarction, post-radiofrequency ablation and more (6).
Clinton Jokerst MD1, Yasmeen Butt MD2, Ann McCullough MD2, Carlos Rojas MD1, Prasad Panse MD1, Kris Cummings MD1, Eric Jensen MD1 and Michael Gotway MD1
Departments of Radiology1
Mayo Clinic Arizona, Scottsdale, AZ USA
Departments of Pathology2
Mayo Clinic Arizona, Scottsdale, AZ USA
References
- Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008 Mar;246(3):697-722. [CrossRef] [PubMed]
- Kuhlman JE, Fishman EK, Siegelman SS. Invasive pulmonary aspergillosis in acute leukemia: characteristic findings on CT, the CT halo sign, and the role of CT in early diagnosis. Radiology. 1985 Dec;157(3):611-4. [CrossRef] [PubMed]
- Wahba H, Truong MT, Lei X, Kontoyiannis DP, Marom EM. Reversed halo sign in invasive pulmonary fungal infections. Clin Infect Dis. 2008 Jun 1;46(11):1733-7. [CrossRef] [PubMed]
- Kim SJ, Lee KS, Ryu YH, Yoon YC, Choe KO, Kim TS, Sung KJ. Reversed halo sign on high-resolution CT of cryptogenic organizing pneumonia: diagnostic implications. AJR Am J Roentgenol. 2003 May;180(5):1251-4. [CrossRef] [PubMed]
- Zompatori M, Poletti V, Battista G, Diegoli M. Bronchiolitis obliterans with organizing pneumonia (BOOP), presenting as a ring-shaped opacity at HRCT (the atoll sign). A case report. Radiol Med. 1999 Apr;97(4):308-10. [PubMed]
- Godoy MC, Viswanathan C, Marchiori E, Truong MT, Benveniste MF, Rossi S, Marom EM. The reversed halo sign: update and differential diagnosis. Br J Radiol. 2012 Sep;85(1017):1226-35. [CrossRef] [PubMed]