March 2022 Medical Image of the Month: Pulmonary Nodules in the Setting of Diffuse Idiopathic Pulmonary NeuroEndocrine Cell Hyperplasia (DIPNECH)
 Wednesday, March 2, 2022 at 8:00AM
Wednesday, March 2, 2022 at 8:00AM 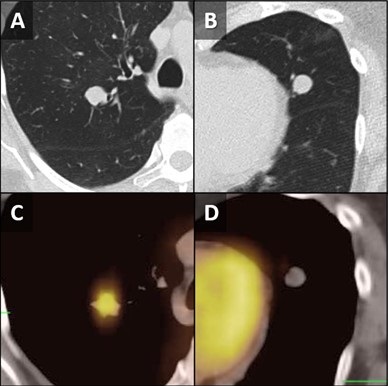
Figure 1. Unenhanced chest CT images in the axial plane show solid, non-calcified and well-circumscribed nodules in the right upper lobe (RUL) (A) and lingula (B). The RUL nodule is FDG-avid on axial fused FDG PET-CT image (C) whereas the lingular nodule is not (D).
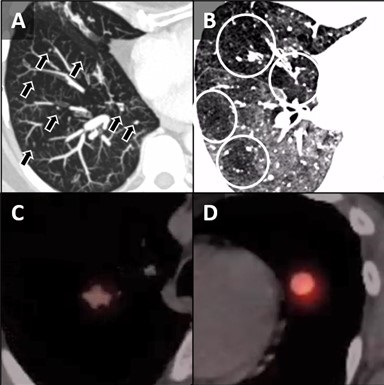
Figure 2. Unenhanced chest CT images in the axial plane reconstructed with maximum intensity projection (MIP, A) and minimum intensity projection (MinIP, B) techniques show multiple scattered solid pulmonary nodules (arrows) and pulmonary mosaicism consistent with air-trapping (circled). Axial fused images from a 68GA-DOTATATE PET-CT demonstrate some activity in the RUL nodule (C) and more prominent uptake in the lingular nodule (D).
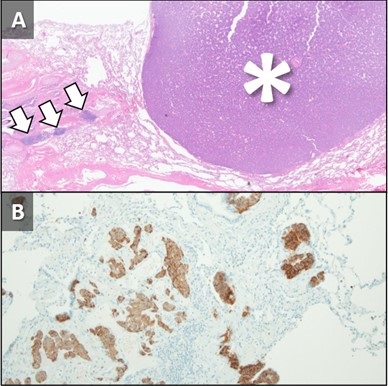
Figure 3. Hematoxylin and Eosin stained low-power pathological image (A) demonstrates the lingular carcinoid tumor (*) as well as several carcinoid tumorlets (arrows) in the adjacent lung. A separate specimen of lung stained with synaptophysin demonstrates multiple tumorlets in the small sample. When taken in conjunction with imaging findings, pathology is in-keeping with a diagnosis of Diffuse Idiopathic Pulmonary Neuroendocrine Cell Hyperplasia (DIPNECH).
A 62-year-old woman presented to an outside hospital with chronic cough, prompting a chest x-ray (CXR). Findings further prompted unenhanced chest CT to evaluate possible pulmonary nodules. The CT demonstrated multiple scattered, solid and centrilobular pulmonary nodules, most of which were small but there were two >1 cm nodules, one in the right upper lobe (RUL) and a second in the lingula (Figure 1A,B). A subsequent FDG PET-CT was performed demonstrating increased metabolic activity in the RUL nodule with no activity in the lingular nodule (Figure 1C,D). Biopsy of the RUL nodule was consistent with a carcinoid. At this point the patient was referred to our center for further management. A repeat chest CT failed to demonstrate any significant change in the nodules. MIP and MinIP reconstructions from that examination demonstrate multiple small, solid pulmonary nodules (arrows) (Figure 2A), many of which were associated with air-trapping resulting in pulmonary mosaicism (circled) (Figure 2B). A 68GA-DOTATATE PET-CT was performed, the results of which provide stark contrast to the FDG-PET in that the RUL nodule demonstrated modest uptake (Figure 2C), whereas the lingular nodule showed very prominent update (Figure 2D). The lingular nodule was resected, H & E-stained pathology image (Figure 3A) demonstrated a typical carcinoid (*) with multiple carcinoid tumorlets in the surgical specimen (arrows). A separate specimen stained with synaptophysin demonstrates multiple neuroendocrine tumorlets. Pathological findings, in conjunction with patient demographics and imaging findings, were consistent with Diffuse Idiopathic Pulmonary NeuroEndocrine Cell Hyperplasia (DIPNECH).
DIPNECH is recognized as a pre-neoplastic lesion in the 2015 WHO classification of lung tumors (1). There is neuroendocrine cell proliferation within the small bronchi and bronchioles which may progress beyond the basement membrane, forming carcinoid tumorlets and in some cases, eventually carcinoid tumors. These airway-centered nodules cause obstruction. In addition, there is often an association between DIPNECH and constrictive bronchiolitis, which causes further airway obstruction (2). The vast majority of patients are women in their 50s-70s and most patients are symptomatic with the most common presenting symptoms being chronic cough and dyspnea (3,4). Many of these patients are often mis-diagnosed with asthma initially (4). The imaging findings of DIPNECH on CT are not specific but can be pathognomonic in some cases. There are almost always innumerable small solid (and sometimes ground glass) centrilobular nodules and nodular bronchial thickening with associated pulmonary mosaicism related to air trapping. Nodules are either stable or very slowly growing over years with the largest nodules usually being biopsied or resected and yielding typical carcinoid on pathology (4). A relatively new nuclear medicine imaging study, 68Ga-DOTATATE PET-CT, shows promise as a higher resolution and more sensitive examination for detection of neuroendocrine tumors (relative to octreotide scans), including pulmonary carcinoid tumors in the setting of DIPNECH (5,6).
Clinton Jokerst MD1, Henry Tazelaar2, Carlos Rojas MD1, Prasad Panse MD1, Kris Cummings MD1, Eric Jensen MD1 and Michael Gotway MD1
Departments of Radiology1 and Pathology2
Mayo Clinic Arizona, Scottsdale, AZ USA
References
- Gosney JR, Austin JHM, Jett J, et al. Diffuse pulmonary neuroendocrine cell hyperplasia. In: Travis WD, Brambilla E, Burke AP, et al., eds. WHO classification of tumours of the lung, pleura, thymus and heart. Lyon, IARC Press, 2015; pp. 78-79.
- Samhouri BF, Azadeh N, Halfdanarson TR, Yi ES, Ryu JH. Constrictive bronchiolitis in diffuse idiopathic pulmonary neuroendocrine cell hyperplasia. ERJ Open Res. 2020 Nov 16;6(4):00527-2020. [CrossRef] [PubMed]
- Rossi G, Cavazza A, Spagnolo P, Sverzellati N, Longo L, Jukna A, Montanari G, Carbonelli C, Vincenzi G, Bogina G, Franco R, Tiseo M, Cottin V, Colby TV. Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia syndrome. Eur Respir J. 2016 Jun;47(6):1829-41. [CrossRef] [PubMed]
- Little BP, Junn JC, Zheng KS, Sanchez FW, Henry TS, Veeraraghavan S, Berkowitz EA. Diffuse Idiopathic Pulmonary Neuroendocrine Cell Hyperplasia: Imaging and Clinical Features of a Frequently Delayed Diagnosis. AJR Am J Roentgenol. 2020 Dec;215(6):1312-1320. [CrossRef] [PubMed]
- Deppen SA, Blume J, Bobbey AJ, Shah C, Graham MM, Lee P, Delbeke D, Walker RC. 68Ga-DOTATATE Compared with 111In-DTPA-Octreotide and Conventional Imaging for Pulmonary and Gastroenteropancreatic Neuroendocrine Tumors: A Systematic Review and Meta-Analysis. J Nucl Med. 2016 Jun;57(6):872-8. [CrossRef] [PubMed]
- Fraum TJ, Ritter JH, Chen DL. Diffuse Idiopathic Pulmonary Neuroendocrine Cell Hyperplasia on Somatostatin Receptor Imaging. Am J Respir Crit Care Med. 2018 Nov 1;198(9):1223-1225. [CrossRef] [PubMed]
Cite as: Jokerst C, Tazelaar H, Rojas C, Panse P, Cummings K, Jensen E, Gotway M. March 2022 Medical Image of the Month: Pulmonary Nodules in the Setting of Diffuse Idiopathic Pulmonary Neuroendocrine Cell Hyperplasia (DIPNECH). Southwest J Pulm Crit Care Sleep;2022:40-42. doi: https://doi.org/10.13175/swjpccs010-22 PDF

Reader Comments