Salma Batool-Anwar, MD, MPH1
Patricia L. Haynes, MPH2
Aria Panchal3
Stuart F. Quan, MD1,2
1Division of Sleep and Circadian Disorders, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA; 2Mel and Enid Zuckerman College of Public Health, University of Arizona, Tucson, AZ, 3University of Toronto, Canada
Abstract
Objectives: Involuntary job loss is a stressful life event that can result in changes in nutritional intake. Both insomnia and obstructive sleep apnea (OSA) also are associated with alterations in dietary intake, but the extent to which this occurs in those who have experienced involuntary job loss is unclear. This study assessed nutritional intake in recently unemployed persons with insomnia and obstructive sleep apnea in comparison to those without a sleep disorder.
Methods: Participants from the Assessing Daily Activity Patterns through Occupational Transitions (ADAPT) study were screened for sleep disorders using the Duke Structured Interview for Sleep Disorders. They were classified as having OSA, acute or chronic insomnia or no sleep disorder. Dietary data was collected using United States Department of Agriculture Multipass Dietary recall methodology.
Results: A total of 113 participants had evaluable data and were included in this study. The cohort was comprised mainly of women (62%) and 24% were non-Hispanic white. Participants with OSA had a higher BMI compared with no sleep disorder (30.6 ± 9.1 vs 27.4 ± 7.1 kg/m2, p≤0.001). Those with acute insomnia had significantly decreased consumption of total protein (61.5 ± 4.7 vs. 77.9 ± 4.9 g, p≤0.05) and total fat (60.0 ± 4.4 vs. 80.5 ± 4.6 g, p≤0.05). Among the participants with chronic insomnia, there was little overall difference in nutrient consumption compared to the no sleep disorder group although there were several gender specific differences. There were no overall differences between participants with OSA in comparison to no sleep disorder, but women consumed less total fat (89.0 ± 6.7 vs. 57.5 ± 8.0 g, p≤0.01). The Healthy Eating Index of all groups was below the average value of Americans.
Conclusion: Unemployed persons compared to those with sleep disorders differ in their consumption of major nutrients; the dietary composition of those with acute insomnia exhibited the greatest divergence. Additionally, the overall nutritional intake of recently unemployed persons is poor.
Key Words: unemployment, insomnia, obstructive sleep apnea, nutrition, diet
Introduction
Sleep is a vital component of healthy living. According to the National Sleep Foundation and the American Academy of Sleep Medicine, 7-8 hours of regular sleep is essential for maintenance and restoration of metabolic homeostasis and to promote optimal health. Unfortunately increasing numbers of people across the globe suffer from sleep deprivation or sleep disorders (1).
Insomnia and obstructive sleep apnea (OSA) are considered health risks and have been linked with cardiovascular diseases (2), increased risk of accidents (3), loss of productivity, worsening metabolic profiles (4), and even premature mortality (5,6). Certain population subgroups comprising of night shift workers, minorities (racial/ethnic), those with fewer years of education or those belonging to lower socioeconomic class (SES) have been shown to be particularly susceptible to several of these adverse consequences.
A major determinant of SES is employment status. Unemployment can result from involuntary job loss, a stressful and disruptive life event. Moreover, joblessness is associated with insomnia symptoms (7), and we have demonstrated an association between recent job loss and OSA (8).
Both insomnia and OSA are associated with differences in nutritional intake in comparison to non-affected persons. Furthermore, we have demonstrated that the quality of nutritional intake is worse in recently unemployed persons (9). However, the impact of insomnia disorder or OSA on nutritional intake has not been evaluated in this population. The Assessing Daily Activity Patterns through Occupational Transitions (ADAPT) Study (10) is an ongoing longitudinal cohort study of individuals who have suffered involuntary job loss in the last 90 days with data collected on the effects of sleep and sleep disorders on nutritional intake and metabolic outcomes. To better understand the immediate health aftermath of job loss, we analyzed cross sectional data from the baseline assessment of the ADAPT study to assess the associations between two sleep disorders, insomnia disorder and OSA, and nutritional intake.
Methods
Participants
Study participants were part of the ADAPT Study, an 18-month longitudinal study that examined changes in sleep, social rhythms, and obesity following an involuntary job loss (10). The study protocol and recruitment strategy have been described in detail previously. Briefly, all individuals who applied for unemployment insurance (UI) in the greater Tucson, Arizona and surrounding areas between October 2015 and December 2018 received study recruitment flyers within their UI intake packets. Interested individuals contacted study staff and completed phone screens assessing exclusion criteria; potentially eligible individuals were then scheduled for in-person screening visits. Individuals were eligible if they had experienced an involuntary job loss within 90 days of study enrollment, had been with their employer for at least six months, were currently employed less than 5 hours per week and did not complete any night shift work within the last 30 days. During the in-person screening, participants provided written informed consent, as well as information about their demographics, employment and medical history. They also were screened for homelessness, existing physiological and mental health conditions, substance abuse, and major sleep diagnoses which could interfere with social rhythms and sleep patterns. Those who passed screening completed validated mental health and sleep diagnostic interviews.
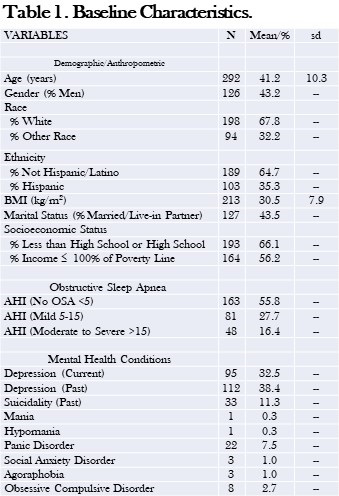
Data used in this analysis originated from the study’s baseline visit. Of the 446 adults who provided written consent, 191 participants met eligibility criteria and completed a baseline assessment visit, including an at-home data collection period lasting two weeks. Participants were considered for the current analysis if there was an acceptable assessment of sleep and diet on their sleep diaries and dietary recalls respectively for analysis. However, 8 participants were excluded as outliers because their mean energy consumption (MEC) was significantly less than commonly reported norms (11, 12). In addition, data for all variables used in analyses were available for only 113 participants. Descriptive statistics for these participants who constitute the study sample are reported in Table 1.
Table 1. Demographic and Anthropometric Characteristics of the Study Population.*
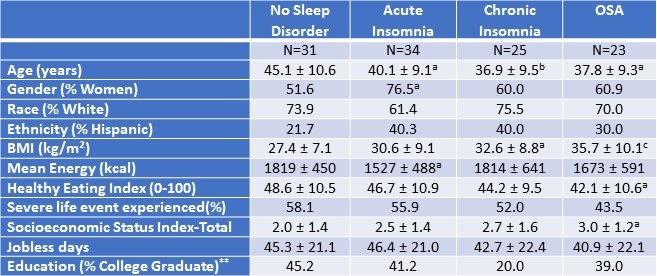 *Data presented as mean ± SD or percentages as appropriate; ap<0.05; bp<0.01; cp<0.001 vs. No Sleep Disorder; **SES assessed as the sum of 4 component scores; education, Income, Employment, and Housing. Click here to open Table 1 in a separate, enlarged window.
*Data presented as mean ± SD or percentages as appropriate; ap<0.05; bp<0.01; cp<0.001 vs. No Sleep Disorder; **SES assessed as the sum of 4 component scores; education, Income, Employment, and Housing. Click here to open Table 1 in a separate, enlarged window.
Measures
Demographic and Anthropometric
Age, ethnicity and biological sex were collected during the initial interview. Height and weight were measured using a stadiometer and bioelectrical impedance analyzer via standardized protocols to calculate the body mass index (kg/m2, BMI). Stressful life events were measured using the Life Events and Difficulties Schedule (LEDS -2) (13), a semi-structured interview and consensus panel rating system in which at least three raters provided contextual assessment of threat associated with different life events over the last three months. All raters were trained and required to achieve a kappa of 0.75 with a trained and reliable rater prior to participation in the rating meeting. Life events were considered severe if they conferred a high degree of threat or unpleasantness during both the immediate aftermath of an event and over the next 10 to 14 days. As in our previous (0=less than high school, 1=high school/some college, 2=bachelor’s degree, 4=postgraduate degree), Income (0=less than or equal to 100% of U.S. poverty line, 1=101-200% of poverty line, 2=201-400% of poverty line, 3=greater than 400% of poverty line), Employment (0=unemployed in last 6 months, 1=employed during last 6 months), and Housing (0=not a homeowner, 1=homeowner).
Diet Assessment
During the two-week, at-home baseline data collection period, participants completed up to three 24-hour dietary recalls administered by trained diet assessors at the Behavioral Measurements and Interventions Shared Resource of the University of Arizona Cancer Center utilizing the gold-standard United States Department of Agriculture Multi-pass Dietary recall (15) and the Nutrient Database System of the University of Minnesota for nutrient analysis (16). These interviews were supported by the Remote Food Photography Method (17), in which participants took pictures of all food and beverages prior to consumption, as well as after they had finished eating and drinking. Photos were used to review recall as a final verification of the multi-pass data. The diet recalls provided information on the types and quantity of food, including energy and nutrient values. At least 3 dietary recalls were completed by 172 participants (95.6% of the entire ADAPT cohort).
Sleep Phenotypes
The Duke Structured Interview for Sleep Disorders (DSISD) (18) was used to classify participants into 4 phenotypes: no sleep disorder (Control), obstructive sleep apnea (OSA), acute insomnia disorder and chronic insomnia disorder. The DSISD is a clinical semi-structured interview developed to assess sleep disorder symptoms and was updated to reflect international classification of sleep disorders (ICSD-3) criteria (19). It is divided into 4 modules respectively focused on insomnia disorders, excessive sleepiness conditions, circadian rhythm disorders and parasomnias. During the interview, participants are asked a series of questions related to possible sleep disturbances. Sections of the questionnaire are skipped if the participant endorses negative answers to screening questions. The DSISD has been validated for classifying persons for OSA (20) and insomnia (18). The DSISD was administered by research staff trained in sleep disorder diagnosis, who met reliability levels of 75% with a licensed clinician (PH).
Statistical Analysis
For baseline characteristics, mean (SD) for continuous variables and percentages for categorical variables were calculated. For multivariate models, estimated marginal means are displayed as mean (SD). The participants without any sleep diagnosis were classified as “No Sleep Disorder”. After classifying participants into sleep phenotypes, comparisons of dietary constituents between No Sleep Disorder and OSA, No Sleep Disorder and acute insomnia and No Sleep Disorder and chronic insomnia groups were performed using analysis of covariance. Models were constructed initially without and subsequently with gender stratification. Included covariates were age, BMI, socioeconomic index, presence of a severe life event as measured by the Life Events and Difficulties schedule. The more liberal rating of severity, short term threat vs long term threat, was used for the purpose of this analysis (long term threat rating of at least 2b). The current variable for severe life events was dichotomized (1 = at least one severe event in the last 3 months; 0 = no severe events in the last 3 months). The level of statistical significance for both models was set at 0.05, but comparisons between 0.05 and 0.10 are provided to illustrate a trend. All statistical analyses were done using STATA version 11 (StataCorp, LLC, College Station, TX, USA) or IBM SPSS version 28 (Armonk, NY).
Results
Table 1 demonstrates the demographic and anthropometric characteristics of the study cohort. Both insomnia groups and the OSA group were younger than controls without a sleep disorder. There was a higher proportion of women in all groups (51.6%, 76.5%, 60%, and 60.9% among participants without any sleep disorder, with acute insomnia, with chronic insomnia, and with OSA respectively). Participants with OSA had a higher BMI compared with no sleep disorder (30.6 ± 9.1 vs 27.4 ± 7.1 kg/m2, p ≤ 0.001). Among the No Sleep Disorder group, 45.2% had college education compared with 20.0% among chronic insomnia, 41.2% in acute insomnia, and 39% among participants with OSA. The participants with acute insomnia had significantly less mean energy consumption in comparison to the control group without a sleep disorder (p<0.05). The proportion of participants who had experienced at least one severe life event was not significantly different amongst the groups.
Table 2 displays mean nutritional intakes for acute insomnia in comparison to controls without a sleep diagnosis stratified by gender.
Table 2. Mean Nutritional Intake--Acute Insomnia Compared to No Sleep Disorder Stratified by Gender.
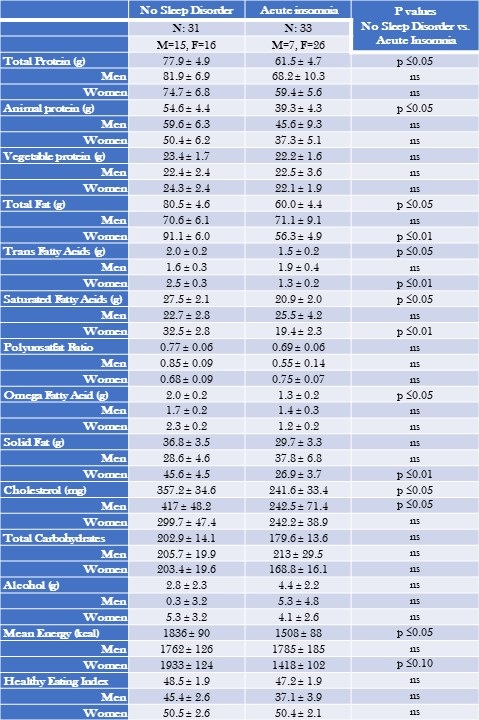 Data are shown as mean ± standard deviation. Non gender and gender stratified models are adjusted for BMI, severity of stress, socioeconomic status, and age. Mean estimates are evaluated at the following values: BMI = 29.3, severity of stress = .58, socioeconomic status total = 2.25, Age = 42.6 years. Click here to view Table 2 in an enlarged, separate window.
Data are shown as mean ± standard deviation. Non gender and gender stratified models are adjusted for BMI, severity of stress, socioeconomic status, and age. Mean estimates are evaluated at the following values: BMI = 29.3, severity of stress = .58, socioeconomic status total = 2.25, Age = 42.6 years. Click here to view Table 2 in an enlarged, separate window.
Acute insomnia was characterized by consumption of lower amounts of total protein (61.5 ± 4.7 vs. 77.9 ± 4.9 g, p≤0.05) and total fat (60.0 ± 4.4 vs. 80.5 ± 4.6, p≤0.05). Reductions in total protein were primarily a result of decreased amounts of animal protein. Decreased total fat was accompanied by lower amounts of trans fatty acids and saturated fatty acids. However, these latter results were driven primarily by lower amounts among women (trans fatty acids: 1.3 ± 0.2 vs 2.5 ± 0.3 g, p≤0.05 among women in comparison to 1.9 ± 0.4 vs 1.6 ± 0.3 g, p=NS among men; saturated fatty acids: 19.4 ± 2.3 vs 32.5 ± 2.8 g, p < 0.01 among women in comparison to 25.5 ± 4.2 vs 22.7 ± 2.8 g, p=NS among men). Similarly, those with acute insomnia consumed less cholesterol compared to those without any sleep disorder (357.2 ± 34.6 vs. 241.6 ± 33.4 mg, p≤0.05). In contrast these results were significant only among men (242.5 ± 71.4 mg vs 417 ± 48.2 g, p<0.05 compared to 242.2 ± 38.9 mg vs 299.7 mg ± 47.4, p= NS among women.
Mean nutritional intakes for chronic insomnia are shown in Table 3.
Table 3: Mean Nutritional Intake--Chronic Insomnia Compared to No Sleep Disorder Stratified by Gender.
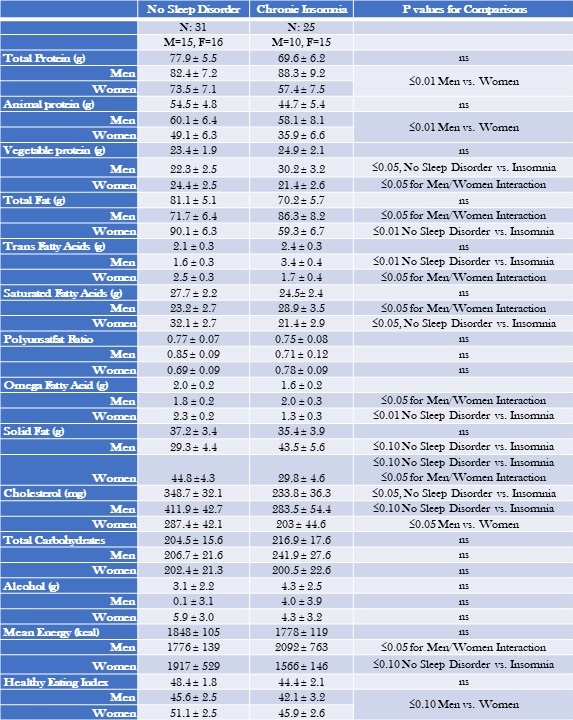 Data are shown as mean ± standard deviation. Non gender and gender stratified models are adjusted for BMI, severity of stress, socioeconomic status, and age. Mean estimates are evaluated at the following values: BMI = 29.7, severity of stress = .55, socioeconomic status = 2.29, Age = 41.4 years. Click here to view Table 3 in an enlarged, separate window.
Data are shown as mean ± standard deviation. Non gender and gender stratified models are adjusted for BMI, severity of stress, socioeconomic status, and age. Mean estimates are evaluated at the following values: BMI = 29.7, severity of stress = .55, socioeconomic status = 2.29, Age = 41.4 years. Click here to view Table 3 in an enlarged, separate window.
There were no overall differences between chronic insomnia and no sleep disorder with the exception of cholesterol which was lower in chronic insomnia (348.7 ± 32.1 vs. 233.8 ± 36.3 mg, p≤0.05). However, after stratification by gender, men consumed more protein and cholesterol irrespective of sleep phenotype. In addition, there were several interactions; men with chronic insomnia consumed more fatty nutrients and women consumed less. Men with chronic insomnia also had greater intake of vegetable protein. (see Table 3 for numeric detail).
Table 4 provides the mean nutritional intake for participants with OSA in comparison to those with no sleep disorder.
Table 4. Mean Nutritional Intake—Obstructive Sleep Apnea (OSA) Compared to No Sleep Disorder Stratified by Gender.
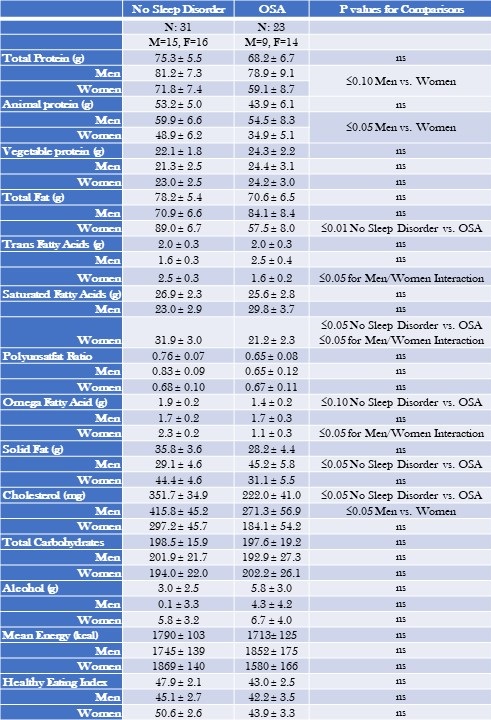 Data are shown as mean ± standard deviation. Gender and Non gender stratified models are adjusted for BMI, severity of stress, socioeconomic status, and age. Mean estimates are evaluated at the following values: BMI = 30.96, severity of stress = .52, socioeconomic status total = 2.39, Age = 41.96 years. Click here to view Table 4 in an enlarged, separate window.
Data are shown as mean ± standard deviation. Gender and Non gender stratified models are adjusted for BMI, severity of stress, socioeconomic status, and age. Mean estimates are evaluated at the following values: BMI = 30.96, severity of stress = .52, socioeconomic status total = 2.39, Age = 41.96 years. Click here to view Table 4 in an enlarged, separate window.
Overall, there were few differences between those with OSA and no sleep disorder. However, less cholesterol was consumed by OSA participants than no sleep disorder (351.7 ± 34.9 vs. 222.0 ± 41.0 mg, p≤0.05). Irrespective of sleep phenotype, women consumed less total protein and animal protein in comparison to men. In addition, women with OSA consumed less fatty nutrients (See Table 4 for numeric detail).
Tables 2, 3, and 4 also show the mean energy intake and Healthy Eating Index for no sleep disorder, acute and chronic insomnia and OSA participants. Participants with acute insomnia consumed fewer calories than controls (1508 ± 88 vs. 1836 ± 90, p≤0.05); this finding was principally observed in women. For chronic insomnia and OSA, there were no overall differences in comparison to controls. However, there was an interaction between phenotype and gender such that women with chronic insomnia had the lowest energy intake. In contrast to an ideal Healthy Eating Index of 100 and the value for the average American diet of 58, no sleep disorder and all three sleep phenotypes had lower values.
Discussion
In this paper, we determined the associations among nutrient intake, acute and chronic insomnia and OSA. We found that participants with acute insomnia had decreased intake of proteins and fats. Among participants with chronic insomnia and OSA, there were few overall differences in dietary intake compared with persons with no sleep disorder. However, for both chronic insomnia and OSA, intake of some nutrients was different from no sleep disorder after gender stratification and also between men and women.
There has been increased interest in diet and sleep quality in recent years; previous epidemiologic studies have demonstrated bidirectional associations between diet quality and sleep (21) (22) (23). The results of our study are consistent with the prior research demonstrating an association between dietary content with sleep quality. Increased sleep efficiency in the elderly has been linked with increased intake of tryptophan (24) which is thought to convert to serotonin, a precursor to melatonin, after crossing the blood brain barrier (25). Katagiri et al (22) demonstrated improved sleep quality as measured by Pittsburgh Sleep Quality Index among participants with high intake of fish and vegetables, whereas poor sleep quality was seen in relation to high consumption of confectionary and noodles.
We observed reductions in protein intake among those with acute insomnia. Previous research has suggested an association between sleep and protein intake. A number of neurotransmitters are known to affect sleep-wake cycle namely 5-HT, gamma aminobutyric acid (GABA), orexin, melanin-concentrating hormone, and histamine(26); dietary precursors can influence the synthesis and function of some of these neurotransmitters. Synthesis of 5-HT is dependent on its precursor availability, the amino acid L tryptophan (Trp)(27). Similar to our results, in a cross-sectional study of non-shift workers, researchers demonstrated an association between low protein intake and poor sleep quality particularly with sleep initiation problems. In contradistinction, in another study of middle-aged Japanese, high protein intake was associated with difficulty maintaining sleep (28). Gao et al also confirmed the differential association of individual insomnia symptoms on nutrition. Using the Health Professional Follow up Study (HPFS), the authors demonstrated difficulty maintaining sleep in relation to a greater energy intake along with an association between difficulty initiating sleep and lower overall diet quality score (29). Unlike our findings, the associations in these latter two studies were limited only to men and did not account for employment status. Although it is difficult to extract a definite conclusion from these studies, there appears to be an effect of protein intake on sleep characteristics.
There are several possible mechanisms that have been proposed to explain the association between diet quality and sleep disorders. One mechanism is that increased hunger and decreased satiety signals lead to orexigenic changes of hunger and fullness (30). Epidemiologic studies have demonstrated lower leptin and higher ghrelin levels among sleep deprived people (31, 32). Another explanation described is related to gastrointestinal discomfort from fullness making it difficult to fall or stay asleep (33). Differential impacts of these mechanisms in different study populations likely contribute to the variability in findings among studies.
Although an association between macroeconomic conditions and mortality and morbidity exists (34) (35), there is paucity of literature analyzing the effect of unemployment, dietary habits, and sleep disorders. Consistent with scant previous research, we observed poor diet quality in the face of involuntary job loss (36). The diet quality as assessed by the Healthy Eating Index (HEI) has been found to have an association with multiple chronic diseases outcomes (37). We found lower scores for HEI among women with chronic insomnia. Although we observed lower fat consumption in persons with acute insomnia, a change of macronutrient composition with an increase in high fat, high sugar, and low fruit and vegetable consumption has been described during stressful times (38)
Economic downturn has been linked with less intake of protein, saturated and total fats and more consumption of carbohydrates (36). Unemployment and financial instability have been shown to lead to unhealthy behaviors on one hand but on the other hand more time is available for healthy food preparation (39). To our knowledge, this study is unique as we examined the association between sleep disorders and diet quality in the setting of involuntary job loss.
We do acknowledge that the cross-sectional nature of this study is a limitation; therefore, we cannot determine causality. Another limitation is that the sample was comprised mainly of women and non- Hispanic whites. As previously described, employment opportunities vary by individual characteristics and particularly ethnicity, and therefore the results of this study may not be generalizable to other populations. Furthermore, persons with confirmed diagnoses of sleep disorders were excluded thus potentially limiting the population to those less impacted by any problems with their sleep. Although the DSISD has previously been validated for classifying OSA, we do acknowledge the limitation of using the structured interview as a surrogate for polysomnography.
In conclusion, the dietary intake of recently unemployed persons with insomnia or OSA is different than those without a sleep disorder. This may reflect the impact of an interaction between the effect of recent job loss and the presence of a sleep disorder on dietary habits. Future longitudinal studies of a racially and ethnically diverse population are needed to better understand the directionality/causality.
Acknowledgements
This work was supported by a grant from the National Heart, Lung and Blood Institute (HL117995).
References
- Institute of Medicine Committee on Sleep, M. and Research, The National Academies Collection: Reports funded by National Institutes of Health, in Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem, H.R. Colten and B.M. Altevogt, Editors. 2006, National Academies Press (US) Copyright © 2006, National Academy of Sciences.: Washington (DC).
- Knutson KL. Sleep duration and cardiometabolic risk: a review of the epidemiologic evidence. Best Pract Res Clin Endocrinol Metab. 2010 Oct;24(5):731-43. [CrossRef] [PubMed]
- Sagaspe P, Taillard J, Bayon V, Lagarde E, Moore N, Boussuge J, Chaumet G, Bioulac B, Philip P. Sleepiness, near-misses and driving accidents among a representative population of French drivers. J Sleep Res. 2010 Dec;19(4):578-84. [CrossRef] [PubMed]
- Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield SB. Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep. 2005 Oct;28(10):1289-96. [CrossRef] [PubMed]
- Gallicchio L, Kalesan B. Sleep duration and mortality: a systematic review and meta-analysis. J Sleep Res. 2009 Jun;18(2):148-58. [CrossRef] [PubMed]
- Grandner MA, Hale L, Moore M, Patel NP. Mortality associated with short sleep duration: The evidence, the possible mechanisms, and the future. Sleep Med Rev. 2010 Jun;14(3):191-203. [CrossRef] [PubMed]
- Maeda M, Filomeno R, Kawata Y, Sato T, Maruyama K, Wada H, Ikeda A, Iso H, Tanigawa T. Association between unemployment and insomnia-related symptoms based on the Comprehensive Survey of Living Conditions: a large cross-sectional Japanese population survey. Ind Health. 2019 Nov 29;57(6):701-710. [CrossRef] [PubMed]
- Silva GE, Quan SF, McMorrow T, Bautista R, Bell ML, Haynes PL. Association between obstructive sleep apnea and multiple involuntary job loss history among recently unemployed adults. Sleep Health. 2021 Feb;7(1):118-122. [CrossRef] [PubMed]
- Batool-Anwar S, Mayer C, Haynes PL, Liu Y, Thomson CA, Quan SF. Impact of Recent Job Loss on Sleep, Energy Consumption and Diet. Southwest J Pulm Crit Care. 2021 Aug 1;23(5):129-137.[CrossRef] [PubMed]
- Haynes PL, Silva GE, Howe GW, Thomson CA, Butler EA, Quan SF, Sherrill D, Scanlon M, Rojo-Wissar DM, Gengler DN, Glickenstein DA. Longitudinal assessment of daily activity patterns on weight change after involuntary job loss: the ADAPT study protocol. BMC Public Health. 2017 Oct 10;17(1):793. [CrossRef] [PubMed]
- Vasquez MM, Goodwin JL, Drescher AA, Smith TW, Quan SF. Associations of dietary intake and physical activity with sleep disordered breathing in the Apnea Positive Pressure Long-Term Efficacy Study (APPLES). J Clin Sleep Med. 2008 Oct 15;4(5):411-8. [PubMed]
- Rock CL, Thornquist MD, Kristal AR, Patterson RE, Cooper DA, Neuhouser ML, Neumark-Sztainer D, Cheskin LJ. Demographic, dietary and lifestyle factors differentially explain variability in serum carotenoids and fat-soluble vitamins: baseline results from the sentinel site of the Olestra Post-Marketing Surveillance Study. J Nutr. 1999 Apr;129(4):855-64. [CrossRef] [PubMed]
- Bifulco A, Brown G, Edwards A, Harris T, Neilson E, Richards C, Robinson R. Life events and difficulties schedule (LEDS-2) Vol. 1: Life events manual. London, England: Royal Holloway and Bedford New College, University of London, 1989.
- Singh V, Haynes PL, Quan SF. Assessing Depression and Suicidality Among Recently Unemployed Persons with Obstructive Sleep Apnea and Socioeconomic Inequality. Southwest J Pulm Crit Care Sleep. 2022 May;24(5):81-88. [CrossRef] [PubMed]
- Subar AF, Thompson FE, Potischman N, Forsyth BH, Buday R, Richards D, McNutt S, Hull SG, Guenther PM, Schatzkin A, Baranowski T. Formative research of a quick list for an automated self-administered 24-hour dietary recall. J Am Diet Assoc. 2007 Jun;107(6):1002-7. [CrossRef] [PubMed]
- Feskanich D, Sielaff BH, Chong K, Buzzard IM. Computerized collection and analysis of dietary intake information. Comput Methods Programs Biomed. 1989 Sep;30(1):47-57. [CrossRef] [PubMed]
- Martin CK, Han H, Coulon SM, Allen HR, Champagne CM, Anton SD. A novel method to remotely measure food intake of free-living individuals in real time: the remote food photography method. Br J Nutr. 2009 Feb;101(3):446-56. [CrossRef] [PubMed]
- Edinger J, Wyatt JK. Reliability and validity of insomnia diagnoses derived from the Duke Structured Interview for Sleep Disorders [abstract]. Sleep, 2009. 32: p. A265.
- Buysse DJ, Reynolds CF 3rd, Kupfer DJ, Thorpy MJ, Bixler E, Manfredi R, Kales A, Vgontzas A, Stepanski E, Roth T, et al. Clinical diagnoses in 216 insomnia patients using the International Classification of Sleep Disorders (ICSD), DSM-IV and ICD-10 categories: a report from the APA/NIMH DSM-IV Field Trial. Sleep. 1994 Oct;17(7):630-7. [CrossRef] [PubMed]
- Silva GE, Rojo-Wissar DM, Quan SF, Haynes PL. Predictive ability of the International Classification of Sleep Disorders-3 in identifying risk of obstructive sleep apnea among recently unemployed adults. Sleep Breath. 2021 Sep;25(3):1325-1334. [CrossRef] [PubMed]
- Fenton S, Burrows TL, Skinner JA, Duncan MJ. The influence of sleep health on dietary intake: a systematic review and meta-analysis of intervention studies. J Hum Nutr Diet. 2021 Apr;34(2):273-285. [CrossRef] [PubMed]
- Katagiri R, Asakura K, Kobayashi S, Suga H, Sasaki S. Low intake of vegetables, high intake of confectionary, and unhealthy eating habits are associated with poor sleep quality among middle-aged female Japanese workers. J Occup Health. 2014;56(5):359-68. [CrossRef] [PubMed]
- Hur S, Oh B, Kim H, Kwon O. Associations of Diet Quality and Sleep Quality with Obesity. Nutrients. 2021 Sep 13;13(9):3181. [CrossRef] [PubMed]
- Bravo R, Matito S, Cubero J, Paredes SD, Franco L, Rivero M, Rodríguez AB, Barriga C. Tryptophan-enriched cereal intake improves nocturnal sleep, melatonin, serotonin, and total antioxidant capacity levels and mood in elderly humans. Age (Dordr). 2013 Aug;35(4):1277-85. [CrossRef] [PubMed]
- Duan KM, Ma JH, Wang SY, Huang Z, Zhou Y, Yu H. The role of tryptophan metabolism in postpartum depression. Metab Brain Dis. 2018 Jun;33(3):647-660. [CrossRef] [PubMed]
- Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005 Oct 27;437(7063):1257-63. [CrossRef] [PubMed]
- Silber BY, Schmitt JA. Effects of tryptophan loading on human cognition, mood, and sleep. Neurosci Biobehav Rev. 2010 Mar;34(3):387-407. [CrossRef] [PubMed]
- Tanaka E, Yatsuya H, Uemura M, Murata C, Otsuka R, Toyoshima H, Tamakoshi K, Sasaki S, Kawaguchi L, Aoyama A. Associations of protein, fat, and carbohydrate intakes with insomnia symptoms among middle-aged Japanese workers. J Epidemiol. 2013;23(2):132-8. [CrossRef] [PubMed]
- Cheng FW, Li Y, Winkelman JW, Hu FB, Rimm EB, Gao X. Probable insomnia is associated with future total energy intake and diet quality in men. Am J Clin Nutr. 2016 Aug;104(2):462-9. [CrossRef] [PubMed]
- St-Onge MP. Sleep-obesity relation: underlying mechanisms and consequences for treatment. Obes Rev. 2017 Feb;18 Suppl 1:34-39. [CrossRef] [PubMed]
- Stern JH, Grant AS, Thomson CA, Tinker L, Hale L, Brennan KM, Woods NF, Chen Z. Short sleep duration is associated with decreased serum leptin, increased energy intake and decreased diet quality in postmenopausal women. Obesity (Silver Spring). 2014 May;22(5):E55-61. [CrossRef] [PubMed]
- Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004 Dec;1(3):e62. [CrossRef] [PubMed]
- Zuraikat FM, Makarem N, Liao M, St-Onge MP, Aggarwal B. Measures of Poor Sleep Quality Are Associated With Higher Energy Intake and Poor Diet Quality in a Diverse Sample of Women From the Go Red for Women Strategically Focused Research Network. J Am Heart Assoc. 2020 Feb 18;9(4):e014587. [CrossRef] [PubMed]
- Ruhm CJ. Are Recessions Good for Your Health? The Quarterly Journal of Economics. 2000; 115(20):617–650. [CrossRef]
- Ruhm CJ. Recessions, healthy no more? J Health Econ. 2015 Jul;42:17-28. [CrossRef] [PubMed]
- Smed S, Tetens I, Bøker Lund T, Holm L, Ljungdalh Nielsen A. The consequences of unemployment on diet composition and purchase behaviour: a longitudinal study from Denmark. Public Health Nutr. 2018 Feb;21(3):580-592. [CrossRef] [PubMed]
- Morze J, Danielewicz A, Hoffmann G, Schwingshackl L. Diet Quality as Assessed by the Healthy Eating Index, Alternate Healthy Eating Index, Dietary Approaches to Stop Hypertension Score, and Health Outcomes: A Second Update of a Systematic Review and Meta-Analysis of Cohort Studies. J Acad Nutr Diet. 2020 Dec;120(12):1998-2031.e15. [CrossRef] [PubMed]
- Habhab S, Sheldon JP, Loeb RC. The relationship between stress, dietary restraint, and food preferences in women. Appetite. 2009 Apr;52(2):437-44. [CrossRef] [PubMed]
- Burgard SA, Ailshire JA, Kalousova L, The Great Recession and health: People, populations, and disparities. The Annals of the American Academy of Political and Social Science. 2013;650(1):194-213. [CrossRef]
Cite as: Batool-Anwar S, Haynes PL, Panchal A, Quan SF. Associations Between Insomnia and Obstructive Sleep Apnea with Nutritional Intake After Involuntary Job Loss. Southwest J Pulm Crit Care Sleep. 2023;26(3):37-47. doi:
https://doi.org/10.13175/swjpccs001-23 PDF
 Saturday, July 1, 2023 at 8:00AM
Saturday, July 1, 2023 at 8:00AM