Sriharsha Rapaka MD 1,2
Priyankar Kumar Datta MD, DNB, DM 3
Shashikant Sharma MD, DM 3,4
1Intensive Care Medicine, St John of God Healthcare, Victoria, Australia
2Critical Care Medicine, All India Institute of Medical Sciences, New Delhi, India
3Anaesthesiology, Pain Medicine and Critical Care, All India Institute of Medical Sciences, New Delhi, India
4Critical Care Medicine, Jayaprabha Medanta Hospital, Patna, India
Abstract
Background: Severe burns can significantly impact various organ systems, including the gastrointestinal (GI) system. GI complications are frequently observed in patients with over 20% total body surface area (TBSA) burn.
Objectives: This case series delves into the intricate phenomenology of post-burn GI dysfunction, challenging conventional cause-and-effect paradigms. Our aim is to discern, comprehend, and explore variables influencing positive and negative outcomes, laying the foundation for further research given the current heterogeneity in the literature.
Methods: Severe burn patients with GI dysfunction identified between April 1, 2022, and July 31, 2022, from the institutional database are included in this retrospective case-series, and comparisons were made across baseline and treatment conditions across participants. Data were collected on demographics, burn characteristics, complications, and treatment outcomes.
Results: We analysed 12 patients with severe burns and GI dysfunction and categorized them into two patterns: Pattern A, characterised by early onset symptoms, gastric and small bowel dilatation, and a relatively benign course with high recovery rates was observed in 6 patients; and Pattern B, characterised by late-onset symptoms, colonic dilatation, shock, and a high mortality rate due to megacolon was seen in 6 patients.
Conclusion: The post-burn GI dysfunction observed in our study is a complex interplay of multiple factors. Adequate fluid resuscitation, timely excision of necrotic tissue, staged food ingestion, specific nutrient administration, and appropriate use of antibiotics and judicious use of selective digestive decontamination (SDD) are essential strategies to prevent and treat this syndrome.
Introduction
Severe burns can have significant physiological impacts on the body, posing a risk to a patient's life that may be exacerbated by complications throughout the stages of treatment (1,2). Gastrointestinal (GI) complications are common in partial and full-thickness burns involving more than 20% TBSA and can include constipation, delayed gastric emptying, bacterial translocation, and sepsis, among others (2,3). While animal models suggest that burns delay gastric emptying and affect gut motility, the exact mechanism in humans is unknown (4,5). Probable causes could include large-volume fluid resuscitation, immobility, increased sympathetic drive secondary to pain, and dietary association with glutamine, opioids, and drugs such as tramadol and tapentadol. This study aims to describe two distinct patterns of bowel dysfunction observed in patients admitted with severe burns and discuss the impact of thermal injuries on gut motility and associated outcomes.
Methodology
Our study includes adult and paediatric patients with severe burns (>20% TBSA) and post-burn GI dysfunction, identified between April 1, 2022, and July 31, 2022. Data collection from discharge codes and chart reviews was conducted independently by two qualified, trained personnel for every participant from the medical records of eligible patients, employing anonymization protocols to uphold patient confidentiality during the entirety of the process. Data, including demographics, burn characteristics, complications, and response to treatment, were collected for the entire course of clinical care and subsequently compiled and reported. The burn unit at the hospital is staffed with highly skilled clinical staff members who have specialized training in treating severe burns. The assessment of treatments and data was supervised by an expert analyst at the faculty level.
Case Descriptions
The long-term outcome of a burn injury dramatically depends on the quality of care received during the initial hours. However, the majority of initial burn care is administered outside of specialized burn centres. It is essential to comprehend the intricacies of Advanced Burn Life Support (ABLS) to ensure the patient's optimal outcome. The medical team provided comprehensive intensive care to manage the patients' GI dysfunction and a description of the management, and treatment approach is summarised below.
- Symptoms: Patients with severe burns presented with symptoms such as diarrhoea, constipation, feed intolerance, abdominal distension, and hypoactive or diminished bowel sounds.
- Workup for diarrhoea: Patients underwent a workup that included testing for C. difficile toxin and stool culture and sensitivity, which both came back negative.
- Treatment for diarrhoea: Patients were treated with oral rehydration solution (ORS), probiotics, and racecadotril capsule (1.5mg/kg). Osmotic diarrhoea mostly resolved with reducing feed volume and protein content. In non-responders with suspected C. difficile infection presenting with fever, leucocytosis and pain abdomen, stool sample for toxin detection or culture was sent and oral metronidazole and, or oral vancomycin therapy was initiated. In patients who progressed to paralytic ileus, IV metronidazole along with oral vancomycin and vancomycin enema were administered.
- Treatment for constipation: Patients received syrup lactulose or syrup sodium picosulfate, liquid paraffin and milk of magnesia. Additionally, prokinetic agents were administered, and if necessary, enemas were used.
- Management of abdominal distension: In cases of abdominal distension, bowel decompression was performed by inserting a nasogastric tube with an intermittent suction system. This procedure aimed to reduce or resolve gastric dilatation, prevent vomiting and decrease the risk of aspiration associated with paralytic ileus.
- Intra-abdominal pressure (IAP) monitoring: Patients with abdominal distension underwent regular IAP monitoring, typically every 4 hours using indirect measurement via the bladder. If IAP exceeded 12 mmHg and was accompanied by hypotension, decreased urine output, or a tense abdomen, more frequent measurements (every 2 hours) were performed. Foley's catheter was also checked for blockage in case of increased IAP values. Monitoring continued until IAP levels dropped below 10 mmHg for several hours, along with clinical improvement.
- Stress ulcer prophylaxis and thromboprophylaxis: Patients above the age of 3 received pantoprazole for stress ulcer prophylaxis. Additionally, adult patients received injection Enoxaparin (1mg/kg) for thromboprophylaxis and mechanical prophylaxis. These measures were continued until patients achieved full ambulation.
- Antibiotics: Antibiotics were initiated only when signs of infection were observed, based on clinical assessment and monitoring of laboratory trends. Once definitive evidence of microbial growth from blood, urine, and wound cultures was obtained, culture-based antibiotics were started.
- Source control: Whenever necessary, the surgical team performed source control procedures to address and manage the underlying cause.
Patient Characteristics
Patients were separated into two patterns based on their clinical characteristics and outcomes (Table 1) and abdominal X-rays (Table 2).
Table 1. Comparison of the Two Patterns of Presentation (to view Table 1 in a new and separate window click here)
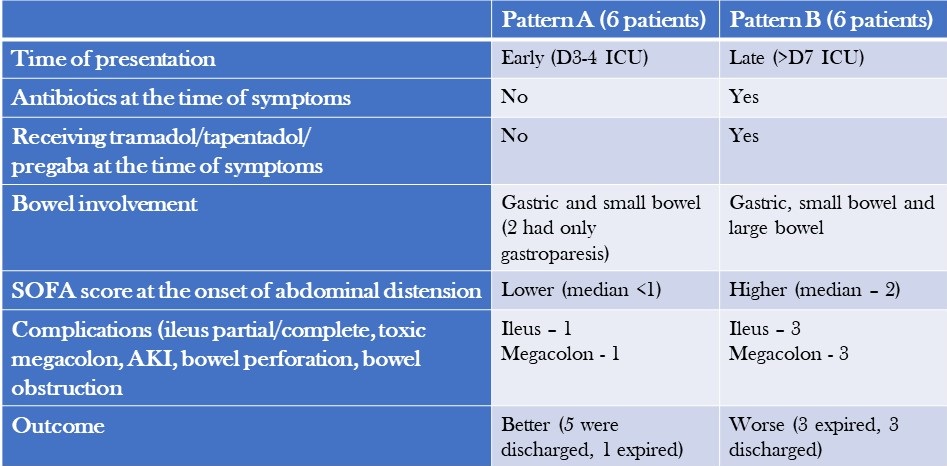
Table 2. Abdominal X-ray Patterns (to view Table 2 in a new and separate window click here)
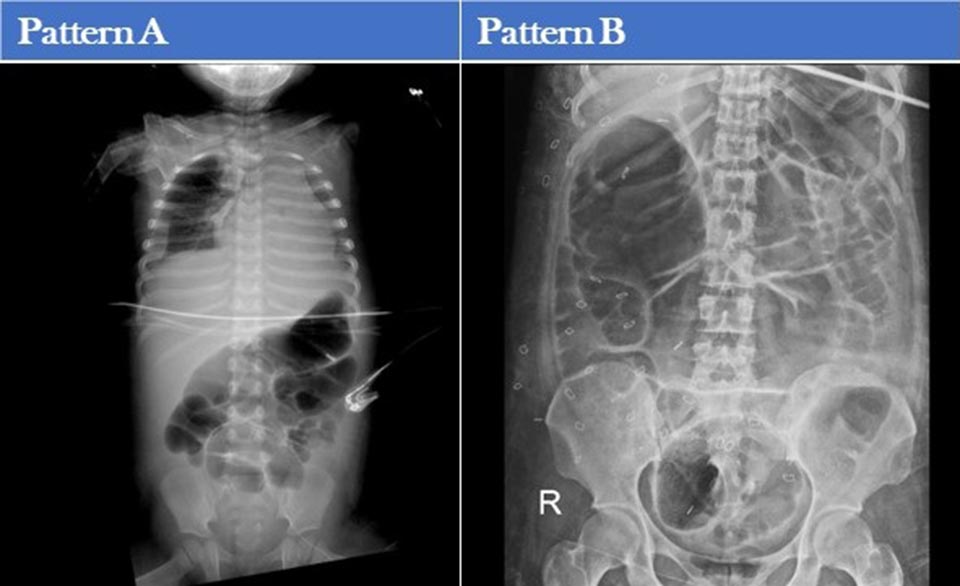
Additional patient characteristics of pattern A and B are shown tables 3 and 4.
Table 3. Clinical Characteristics, Laboratory, and Imaging Findings of Patients with Pattern A GI Dysfunction (to view Table 3 in a new and separate window click here)
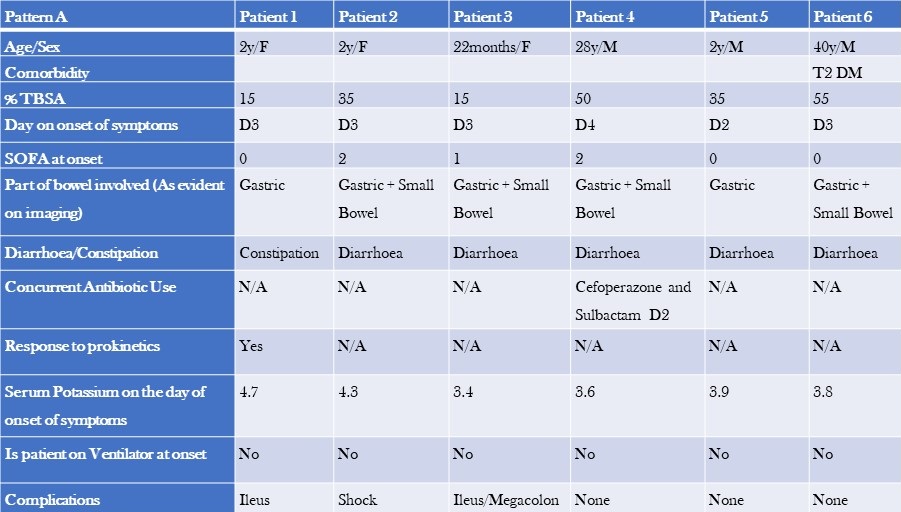
- TBSA=total burn surface area
- SOFA=Sequential Organ Failure Assessment Score
Table 4. Clinical Characteristics, Laboratory, and Imaging Findings of Patients with Pattern B GI Dysfunction (to view Table 4 in a new and separate window click here)
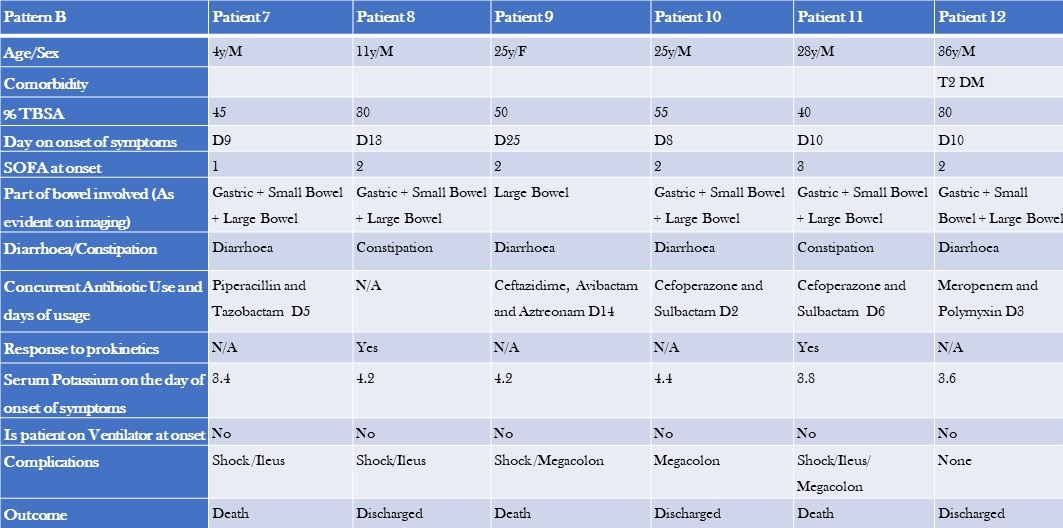
The two groups differed in baseline characteristics. The first group had a smaller median TBSA compared to the second group (32.5% vs 42.5%). Additionally, the first group comprised primarily paediatric patients, and their GI dysfunction developed earlier (median day 3 vs day 10), with a lower median SOFA score (0 vs 1). The second group had colonic dilatation in addition to gastric and small bowel dilatation, and all patients had signs and, or evidence of infection and were on antibiotics by the time they developed GI dysfunction. The median serum potassium levels were also slightly different between the two groups (3.8 vs 4.2). Notably, there were more deaths in the second group (50%) compared to the first group, where most patients recovered and were shifted to a step-down unit.
Discussion
The stress response, metabolic changes, and nutritional deficiencies primarily cause most gastrointestinal (GI) issues associated with burn injuries. If not promptly recognized and appropriately treated, these complications can lead to severe consequences, including fatal haemorrhage or perforation. Implementing early prophylactic measures during the post-burn period is crucial to prevent these outcomes. One common complication of thermal injuries is gastric distention and dysfunction.
Studies have shown that gastric emptying is significantly reduced by approximately 37-42% at 6 hours after a burn (4-6). In our study, we observed early gastric dilation upon admission. Burn injuries also affect the standard slow wave frequency in the stomach, increasing the occurrence of bradygastria (7). However, patients who arrived at the emergency department within 2 hours of the burn injury and received timely resuscitation mostly remained asymptomatic. Radiological evidence revealed gastric dilation, which eventually resolved during their hospital stay.
Animal studies have demonstrated that small intestinal transit time is significantly decreased in burn injury models compared to control groups at 2 hours (8,9) and 6 hours post-burn (5,6,9,10). In our study, we observed early small bowel dilation and ileus during the ICU stay. Chen et al conducted a study with rat models, revealing that the gastrointestinal motility in burn-injured rats treated with saline is notably higher compared to untreated burn-injured rats (11). This finding aligns with our observations, as most patients who arrived early and received timely resuscitation showed resolution of bowel dilation.
Colonic transit time was delayed compared to the control group in burn injury patients (5,12). We could not find any literature on this topic in human subjects, highlighting the need for prospective studies. We noticed colonic involvement in symptomatic patients approximately one week after the burn injury. In cases of severe abdominal distension, dilated bowel loops, and feed intolerance, supplemental parenteral nutrition/TPN was administered. Early fluid resuscitation within 2 hours of a thermal injury is crucial in preventing multiple organ failure and mortality (18).
As described above, "Pattern A" patients experienced early symptoms during their ICU stay, showed minimal signs of infection, and had a relatively milder course with a lower mortality rate compared to "Pattern B" patients. Pattern B patients presented later, experienced more complications, and had higher morbidity and mortality rates. Dysmotility in these patients could be attributed to sepsis, opioids, or antibiotics. We tested for C. difficile toxin and culture, which came back negative. Immobility, opioid use, pain, and dietary glutamine are common causes of GI dysfunction in both patient groups. Incremental fentanyl infusion was administered to all patients within 24-48 hours of the injury. Breakthrough and procedural pain were managed with sub-anaesthetic doses of IV ketamine and IV fentanyl. Patients presenting with Pattern B symptoms were often prescribed slow-release oral tramadol/oral tapentadol/ pregabalin formulations to supplement or replace opioids due to concerns about constipation, tolerance, and addiction. Opioids could exacerbate GI symptoms like vomiting and constipation (14). Tramadol was found to delay colonic transit but did not affect upper gastrointestinal transit.15 Tapentadol, on the other hand, provided analgesia with a more tolerable side effect profile and resulted in less deterioration of gastrointestinal function and symptoms compared to standard opioids (16,17). However, results from different studies on tapentadol’ s effects on gastric emptying and bowel function are inconsistent, making its routine use in severe burns unclear (18,19). NSAIDs are effective for mild to moderate burns, but opioids are preferred in severe cases due to acute kidney injury (AKI) concerns. AKI is common in severe burns and an independent mortality risk factor. While opioids and NSAIDs may have contributed to large bowel dysmotility in Pattern B patients, a causal relationship cannot be established.
Burn-injured patients often experience acute and chronic neuropathic pain. Pregabalin has shown efficacy in reducing neuropathic pain and improving sleep but may cause constipation (20,21). Stress ulcer prophylaxis with pantoprazole was administered to patients above three years of age. Short-term treatment with proton pump inhibitors (PPIs) has been reported to delay gastric emptying of solid meals in healthy individuals (22). The effects of PPIs on liquid emptying are inconsistent (23). Prolonged gastric residence of PPIs due to delayed emptying may impact their pharmacological effectiveness, which can be clinically relevant in managing conditions such as GERD, functional dyspepsia, and diabetes (24). However, routine administration of PPIs in severe burn patients is not recommended. Although a systematic review and meta-analysis suggested a potential correlation between the usage of proton pump inhibitors (PPIs) and a heightened likelihood of contracting Clostridium difficile infection (CDI), we did not find any substantiating evidence of CDI (25). Further high-quality and prospective studies are needed to establish a causal relationship.
Major burns trigger an inflammatory response and catabolism, which can lead to severe nutrition deficiencies when combined with burn wound nutrient losses. These deficiencies can impair immune function and wound healing and increase the risk of organ injury and mortality (26). Sepsis causes dysbiosis and bacterial translocation (27). Severe burn patients frequently experience sepsis-induced ileus (28). Early and staged enteral nutrition has been shown to reduce gram-negative bacteraemia in burn patients and promote a healthy intestinal microenvironment (29-32). Caloric requirements were calculated using the Curreri formula for adults and Curreri junior formula for paediatric patients. However, as the formula often overestimates caloric needs, a target of 70-80% of the calculated requirement was set. Using continuous feeding bags, oral and/or nasogastric feeding was initiated from day 1 in the ICU. Post-pyloric feeding was administered to patients with feed intolerance or high gastric residual volume. Micronutrients and trace elements were supplemented, and glutamine and fibre were added to the diet for adult patients. Glutamine stimulates the release of glucagon-like peptide-1, which increases postprandial insulin secretion and slows gastric emptying (33). Current recommendations support using glutamine in severe burn patients due to promising evidence and minimal adverse effects. The RE-ENERGIZE trial showed mortality at 6 months was 17.2% in the glutamine group and 16.2% in the placebo group (hazard ratio for death, 1.06; 95% CI, 0.80 to 1.41) and no substantial between-group differences in serious adverse events (26).
We hypothesize that prudent utilization of selective digestive decontamination (SDD) may reduce infections and improve survival in severe burn patients (34). In a randomized trial, SDD demonstrated improved survival. However, according to a meta-analysis, enteral antibiotic use did not reduce mortality in severe burn patients, which aligns with our findings (35).
Managing wounds in the early stages and providing postoperative care after skin grafting pose challenges in patients with extensive burns. Effective use of negative pressure wound therapy (NPWT) can facilitate better wound healing and reduce infections. Patients with burns involving the perineum and genitalia present particular challenges due to increased wound infections, graft loss, and sepsis caused by dressing soiling (36-38). We hypothesize that faecal management systems might reduce infections by diverting faeces and improving personal hygiene in severe burn patients. A retrospective study found a survival benefit with no significant complications associated with faecal management systems (39).
Limitations
Our study is a retrospective case series that has inherent constraints. Our study lacked a control group. Selection bias and treatment assignment bias cannot be ruled out. These unregulated and unidentified factors of variation have the potential to influence the general applicability of the study's outcomes. Further prospective studies are needed to establish causal associations.
Conclusions
The first pattern of patients, primarily children without underlying health conditions, appeared to have experienced bowel dysfunction as a stress response amplified using PPIs. Diarrhoea in these cases was not due to an infection, and excessive sympathetic activity could be the contributing factor. On the other hand, the second pattern of patients, primarily adults with comorbidities, were seriously ill and received a combination of antibiotics, opioids, and gabapentin. These patients were also experiencing sepsis and sepsis-induced ileus, which is common in individuals with severe burns. In this group, diarrhoea could be caused by an infectious or non-infectious agent, and while testing for C. difficile was negative, there may have been delays in the transportation and analysis of stool samples that resulted in false negative results. It is important to note that repeating the tests is unlikely to improve the sensitivity of the results (40).Top of Form
Learning Points
- Post-burn gastrointestinal issues are caused by a combination of factors that disrupt the balance of gut microbes leading to sepsis and multiple organ dysfunction syndrome (MODS).
- Further prospective studies are needed to establish the effect of tramadol, tapentadol and pregabalin on GI system in severe burns.
- The regular use of PPIs may worsen the impact of severe burns on the gut.
- Managing serious burns necessitates a collaborative strategy encompassing prompt and effective fluid replacement, timely removal of deceased tissue, cautious initiation of nutrition, targeted use of antibiotics, and thoughtful application of selective digestive decontamination (SDD) to prevent gastrointestinal complications and reduce mortality.
- Faecal management systems and negative pressure wound therapy (NPWT) can help to improve wound care and hygiene in patients with perineal burns.
References
- Jeschke MG, Pinto R, Kraft R, Nathens AB, Finnerty CC, Gamelli RL, Gibran NS, Klein MB, Arnoldo BD, Tompkins RG, Herndon DN; Inflammation and the Host Response to Injury Collaborative Research Program. Morbidity and survival probability in burn patients in modern burn care. Crit Care Med. 2015 Apr;43(4):808-15. [CrossRef] [PubMed]
- Jonason AM. Complications of burn injury. Occup Health Nurs. 1983 Jul;31(7):24-8. [CrossRef] [PubMed]
- Czaja AJ, McAlhany JC, Pruitt BA Jr. Acute gastroduodenal disease after thermal injury. An endoscopic evaluation of incidence and natural history. N Engl J Med. 1974 Oct 31;291(18):925-9. [CrossRef] [PubMed]
- Sallam HS, Kramer GC, Chen JD. Gastric emptying and intestinal transit of various enteral feedings following severe burn injury. Dig Dis Sci. 2011 Nov;56(11):3172-8. [CrossRef] [PubMed]
- Sallam HS, Oliveira HM, Gan HT, Herndon DN, Chen JD. Ghrelin improves burn-induced delayed gastrointestinal transit in rats. Am J Physiol Regul Integr Comp Physiol. 2007 Jan;292(1):R253-7. [CrossRef] [PubMed]
- Oliveira HM, Sallam HS, Espana-Tenorio J, Chinkes D, Chung DH, Chen JD, Herndon DN. Gastric and small bowel ileus after severe burn in rats: the effect of cyclooxygenase-2 inhibitors. Burns. 2009 Dec;35(8):1180-4. [CrossRef] [PubMed]
- Sallam HS, Oliveira HM, Liu S, Chen JD. Mechanisms of burn-induced impairment in gastric slow waves and emptying in rats. Am J Physiol Regul Integr Comp Physiol. 2010 Jul;299(1):R298-305. [CrossRef] [PubMed]
- Alican I, Coşkun T, Yeğen C, Aktan AO, Yalin R, Yeğen BC. The effect of thermal injury on gastric emptying in rats. Burns. 1995 May;21(3):171-4. [CrossRef] [PubMed]
- Unlüer EE, Alican I, Yeğen C, Yeğen BC. The delays in intestinal motility and neutrophil infiltration following burn injury in rats involve endogenous endothelins. Burns. 2000 Jun;26(4):335-40. [CrossRef] [PubMed]
- Oktar BK, Cakir B, Mutlu N, Celikel C, Alican I. Protective role of cyclooxygenase (COX) inhibitors in burn-induced intestinal and liver damage. Burns. 2002 May;28(3):209-14. [CrossRef] [PubMed]
- Chen CF, Chapman BJ, Munday KA, Fang HS. The effects of thermal injury on gastrointestinal motor activity in the rat. Burns Incl Therm Inj. 1982 Nov;9(2):142-6. [CrossRef] [PubMed]
- Gan HT, Chen JD. Roles of nitric oxide and prostaglandins in pathogenesis of delayed colonic transit after burn injury in rats. Am J Physiol Regul Integr Comp Physiol. 2005 May;288(5):R1316-24. [CrossRef] [PubMed]
- Barrow RE, Jeschke MG, Herndon DN. Early fluid resuscitation improves outcomes in severely burned children. Resuscitation. 2000 Jul;45(2):91-6. [CrossRef] [PubMed]
- Melchior C, Desprez C, Wuestenberghs F, Leroi AM, Lemaire A, Goucerol G. Impact of Opioid Consumption in Patients With Functional Gastrointestinal Disorders. Front Pharmacol. 2020 Dec 21;11:596467. [CrossRef] [PubMed]
- Wilder-Smith CH, Bettiga A. The analgesic tramadol has minimal effect on gastrointestinal motor function. Br J Clin Pharmacol. 1997 Jan;43(1):71-5. [CrossRef] [PubMed]
- Singh DR, Nag K, Shetti AN, Krishnaveni N. Tapentadol hydrochloride: A novel analgesic. Saudi J Anaesth. 2013 Jul;7(3):322-6. [CrossRef] [PubMed]
- Etropolski M, Kelly K, Okamoto A, Rauschkolb C. Comparable efficacy and superior gastrointestinal tolerability (nausea, vomiting, constipation) of tapentadol compared with oxycodone hydrochloride. Adv Ther. 2011 May;28(5):401-17. [CrossRef] [PubMed]
- Mark EB, Nedergaard RB, Hansen TM, Nissen TD, Frøkjaer JB, Scott SM, Krogh K, Drewes AM. Tapentadol results in less deterioration of gastrointestinal function and symptoms than standard opioid therapy in healthy male volunteers. Neurogastroenterol Motil. 2021 Nov;33(11):e14131. [CrossRef] [PubMed]
- Jeong ID, Camilleri M, Shin A, et al. A randomised, placebo-controlled trial comparing the effects of tapentadol and oxycodone on gastrointestinal and colonic transit in healthy humans. Aliment Pharmacol Ther. 2012 May;35(9):1088-96. [CrossRef] [PubMed]
- Gray P, Kirby J, Smith MT, Cabot PJ, Williams B, Doecke J, Cramond T. Pregabalin in severe burn injury pain: a double-blind, randomised placebo-controlled trial. Pain. 2011 Jun;152(6):1279-1288. [CrossRef] [PubMed]
- Toth C. Pregabalin: latest safety evidence and clinical implications for the management of neuropathic pain. Ther Adv Drug Saf. 2014 Feb;5(1):38-56. [CrossRef] [PubMed]
- Kurt A, Altun A, Bağcivan I, Koyuncu A, Topcu O, Aydın C, Kaya T. Effects of proton pump inhibitors and h(2) receptor antagonists on the ileum motility. Gastroenterol Res Pract. 2011;2011:218342. [CrossRef] [PubMed]
- Sanaka M, Yamamoto T, Kuyama Y. Effects of proton pump inhibitors on gastric emptying: a systematic review. Dig Dis Sci. 2010 Sep;55(9):2431-40. [CrossRef] [PubMed]
- Baron JH. The pharmacology of gastric acid. Scand J Gastroenterol Suppl. 1983;18(87):7-23.
- Trifan A, Stanciu C, Girleanu I, Stoica OC, Singeap AM, Maxim R, Chiriac SA, Ciobica A, Boiculese L. Proton pump inhibitors therapy and risk of Clostridium difficile infection: Systematic review and meta-analysis. World J Gastroenterol. 2017 Sep 21;23(35):6500-6515. [CrossRef] [PubMed]
- Wischmeyer PE. Glutamine in Burn Injury. Nutr Clin Pract. 2019 Oct;34(5):681-687. [CrossRef] [PubMed]
- Fay KT, Ford ML, Coopersmith CM. The intestinal microenvironment in sepsis. Biochim Biophys Acta Mol Basis Dis. 2017 Oct;1863(10 Pt B):2574-2583. [CrossRef] [PubMed]
- Kirksey TD, Moncrief JA, Pruitt BA Jr, O'Neill JA Jr. Gastrointestinal complications in burns. Am J Surg. 1968 Nov;116(5):627-33. [CrossRef] [PubMed]
- Huang HH, Lee YC, Chen CY. Effects of burns on gut motor and mucosa functions. Neuropeptides. 2018 Dec;72:47-57. [CrossRef] [PubMed]
- He W, Wang Y, Wang P, Wang F. Intestinal barrier dysfunction in severe burn injury. Burns Trauma. 2019 Jul 26;7:24. [CrossRef] [PubMed]
- Earley ZM, Akhtar S, Green SJ, et al. Burn Injury Alters the Intestinal Microbiome and Increases Gut Permeability and Bacterial Translocation. PLoS One. 2015 Jul 8;10(7):e0129996. [CrossRef} [PubMed]
- Xiao SC, Zhu SH, Xia ZF, Lu W, Wang GQ, Ben DF, Wang GY, Cheng DS. Prevention and treatment of gastrointestinal dysfunction following severe burns: a summary of recent 30-year clinical experience. World J Gastroenterol. 2008 May 28;14(20):3231-5. [CrossRef] [PubMed]
- Du YT, Piscitelli D, Ahmad S, Trahair LG, Greenfield JR, Samocha-Bonet D, Rayner CK, Horowitz M, Jones KL. Effects of Glutamine on Gastric Emptying of Low- and High-Nutrient Drinks in Healthy Young Subjects-Impact on Glycaemia. Nutrients. 2018 Jun 7;10(6):739. [CrossRef] [PubMed]
- de La Cal MA, Cerdá E, García-Hierro P, van Saene HK, Gómez-Santos D, Negro E, Lorente JA. Survival benefit in critically ill burned patients receiving selective decontamination of the digestive tract: a randomized, placebo-controlled, double-blind trial. Ann Surg. 2005 Mar;241(3):424-30. [CrossRef] [PubMed]
- Rubio-Regidor M, Martín-Pellicer A, Silvestri L, van Saene HKF, Lorente JA, de la Cal MA. Digestive decontamination in burn patients: A systematic review of randomized clinical trials and observational studies. Burns. 2018 Feb;44(1):16-23. [CrossRef] [PubMed]
- Gómez-Ortega V, Vergara-Rodriguez MJ, Mendoza B, García T. Effect of Negative Pressure Wound Therapy in Electrical Burns. Plast Reconstr Surg Glob Open. 2021 Feb 17;9(2):e3383. [CrossRef] [PubMed]
- Teng SC. Use of negative pressure wound therapy in burn patients. Int Wound J. 2016 Sep;13 Suppl 3(Suppl 3):15-8. [CrossRef] [PubMed]
- Kantak NA, Mistry R, Varon DE, Halvorson EG. Negative Pressure Wound Therapy for Burns. Clin Plast Surg. 2017 Jul;44(3):671-677. [CrossRef] [PubMed]
- Farroha A, Frew Q, Philp B, Dziewulski P. Improvement of survival in patients with extensive burns involving the perineum with use of a faecal management system. Ann Burns Fire Disasters. 2014 Mar 31;27(1):14-6. [PubMed]
- Bagdasarian N, Rao K, Malani PN. Diagnosis and treatment of Clostridium difficile in adults: a systematic review. JAMA. 2015 Jan 27;313(4):398-408. [CrossRef] [PubMed]
Cite as:
Rapaka S, Datta PK, Sharma S. Delineating Gastrointestinal Dysfunction Variants in Severe Burn Injury Cases: A Retrospective Case Series with Literature Review. Southwest J Pulm Crit Care Sleep. 2024;28(3):40-48. doi:
https://doi.org/10.13175/swjpccs006-24 PDF
 Monday, April 1, 2024 at 8:00AM
Monday, April 1, 2024 at 8:00AM