Christopher S Dossett MD1, Kelli Kosako Yost MD1, Christopher Lau MD2, Nafis Shamsid-Deen MD2
1Department of Internal Medicine, University of Arizona – Phoenix
2Department of Pulmonary and Critical Care Medicine, University of Arizona – Phoenix
Address: 475 N. 5th Street, Phoenix, Arizona, United States of America
Abstract
Bleomycin is a common chemotherapy agent used to treat germinative tumors. Bleomycin-induced lung injury (BILI) is an uncommon but devastating adverse effect of its use. It occurs in 10-20% of patients receiving bleomycin, and the initial diagnosis is usually made by new-onset respiratory symptoms and reduced diffusing capacity for carbon monoxide (DLCO). Mainstay treatment includes discontinuing bleomycin, corticosteroids, and supplemental oxygen if needed. We present a case of a 38-year-old male who was found to have a severe presentation of bleomycin-induced lung injury after chemotherapy for metastatic mixed germ cell testicular cancer. During his course, he was treated with the standard of care regimen of corticosteroids and salvage therapy with infliximab but ultimately died from complications of his illness. This case report is noteworthy because our patient had progressive bleomycin-induced lung injury, despite discontinuing bleomycin many months prior, consistent high-dose corticosteroid treatment, and even salvage therapy. In all patients on bleomycin, pulmonary function monitoring is essential, and any complaints of dyspnea should prompt concern for bleomycin-induced lung injury. If initial treatment does not improve their condition, more aggressive measures may be necessary.
Abbreviations
- ARDS - acute respiratory distress syndrome
- BILI - bleomycin-induced lung injury
- CT - computed tomography
- DLCO - diffusing capacity for carbon monoxide
- ECMO - extracorporeal membrane oxygenation
- FDA - Food and Drug Administration
- IU - international units
- PFT - pulmonary function tests
- ROS - reactive oxygen species
Introduction
Bleomycin is an antibiotic used to treat germinative tumors and Hodgkin’s lymphoma. The major limitation of bleomycin therapy is pulmonary toxicity, which occurs in up to 10-20% of patients receiving the drug, with mortality up to 1-2% (1). The primary mechanism is not entirely understood but is thought to be induced by the generation of reactive oxygen species (ROS) that form free radical oxidants (2). When type I pneumocytes experience oxidation from free radicals, they undergo apoptosis. This release of cellular contents can lead to the activation of neutrophils and pulmonary macrophages. These cells release cytokines and chemokines, which attract more inflammatory cells, amplifying the immune response. This ultimately disrupts the alveolar-capillary interface, causing capillary leak. This inflammation stimulates fibroblasts resulting in collagen deposits and irreversible pulmonary fibrosis.
The mainstay treatment of bleomycin-induced lung injury (BILI) involves discontinuing the medication and initiating corticosteroids to reduce inflammation (3). There has been limited updated evidence on managing BILI since White and Stover (3) in 1984 noted clinical improvement with corticosteroids. Only case series and reports have provided additional clinical experience on the efficacy of this treatment (4-7). As corticosteroids are helpful in acute BILI, patients with more indolent disease may benefit less. Recent case reports have trailed off-label therapies, including tumor necrosis alpha inhibitors, tyrosine kinase inhibitors, and antifibrotics, as potential treatment options with mixed results (8-12). Despite this well-known adverse effect of bleomycin, minimal evidence-based changes have been made in managing BILI, especially when refractory to corticosteroids. We present a case of a patient who developed rapidly progressive bleomycin-induced lung injury despite discontinuing bleomycin, initiation of high-dose corticosteroids, and salvage infliximab therapy.
Case Presentation
A 38-year-old man with a 10-pack-year tobacco use history and metastatic mixed germ cell testicular cancer undergoing bleomycin, etoposide, and cisplatin chemotherapy, with his last treatment a month prior, presented to a nearby emergency department with shortness of breath. He had completed four chemotherapy treatment cycles initiated three months earlier for a combined bleomycin dose of 330,000 IU (330 milligrams). Baseline pulmonary function test (PFT) before initiation of bleomycin showed a normal diffusing capacity for carbon monoxide (DLCO) at 90% of predicted. In the emergency department, he was found to be in respiratory failure with new onset ground-glass opacifications throughout bilateral lung fields by computed tomography (CT) angiogram (Figure 1).
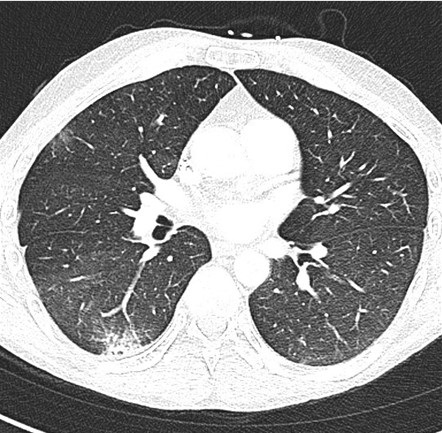 Figure 1. Initial presentation CT pulmonary angiogram demonstrating ground-glass opacities present throughout both lungs. To view Figure 1 in a separate, enlarged window click here.
Figure 1. Initial presentation CT pulmonary angiogram demonstrating ground-glass opacities present throughout both lungs. To view Figure 1 in a separate, enlarged window click here.
He denied using any vaping products. Infectious work-up was negative for SARS-CoV-2, influenza, and coccidiosis. He was treated for community-acquired pneumonia with an initial improvement of his respiratory failure and discharged a few days later on ambient air.
Despite oral antibiotic therapy and stopping cigarette use after discharge, the patient’s dyspnea and cough recurred less than a week after hospitalization. Repeat PFTs demonstrated new findings of reduced DLCO at 31% of predicted. Bleomycin was discontinued from his chemotherapy regimen due to concern of BILI. He was started on daily prednisone 60 mg, or approximately 1 mg/kg. He was tapered to 40 mg of prednisone daily over four weeks, but due to worsening dyspnea symptoms it had to be increased to 50 mg daily.
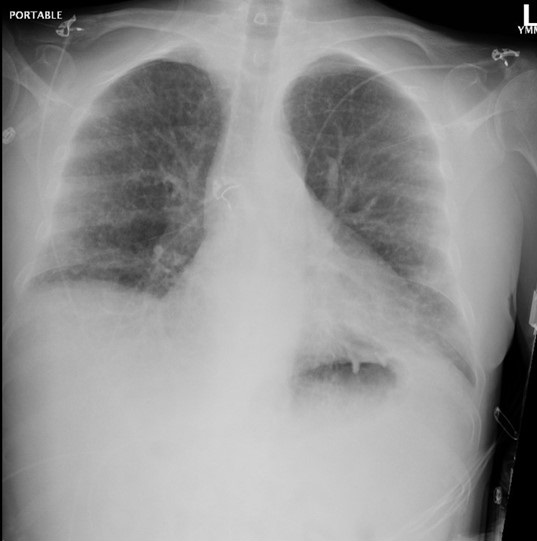
The patient re-presented to the emergency department one month after his initial hospitalization for acute on chronic shortness of breath and a persistent cough. Between these hospitalizations, the patient had not received etoposide or cisplatin treatment. His heart rate was 111 beats per minute, his respiratory rate was 16 breaths per minute, and his oxygen saturation was 95% at ambient air. Laboratory data was mainly unremarkable, except for a white blood cell count of 18.4 K/uL with neutrophilic predominance at 16.23 K/uL, hemoglobin 10.7 g/dL with an MCV of 103 fL, and C-reactive protein of 38.1 mg/L. A CT pulmonary angiogram demonstrated worsening interstitial and airspace opacities (Figure 2).
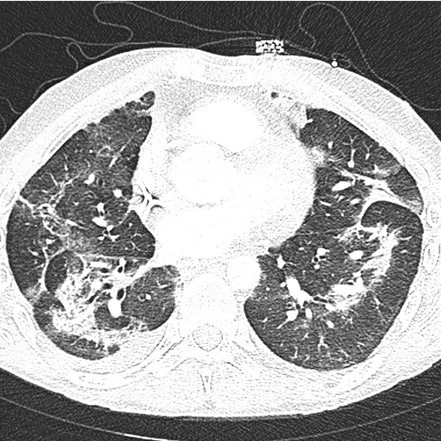 Figure 2. CT pulmonary angiogram shows significant interstitial and airspace opacity progression throughout the lungs. To view Figure 2 in a separate, enlarged window click here.
Figure 2. CT pulmonary angiogram shows significant interstitial and airspace opacity progression throughout the lungs. To view Figure 2 in a separate, enlarged window click here.
He was admitted and treated with broad-spectrum antibiotics due to concern of recurrent pneumonia, as he was not on antibiotic prophylaxis with his chronic steroids. The patient was resumed on his outpatient dose of oral prednisone 50 mg daily. Infectious work-up including blood, sputum, and fungal cultures, legionella antibodies, streptococcus pneumonia urinary antigen, mycoplasma antibodies, aspergillus, fungitell, coccidioides serologies, HIV, and viral etiologies including SARS-CoV-2, influenza, and cytomegalovirus were all unremarkable. Due to a broad negative infectious work-up, BILI was highly thought to be the original diagnosis. He was switched to intravenous methylprednisolone 60 mg every 12 hours for more aggressive BILI treatment.
Two days after admission, he became acutely dyspneic. A repeat chest radiographic demonstrated continued bilateral airspace opacities with new moderate to large right apical pneumothorax. He underwent CT-guided right thoracostomy tube placement for the new pneumothorax. His respiratory status deteriorated over the next five days requiring endotracheal intubation. Bronchoalveolar lavage performed during intubation was unremarkable for infectious etiologies or malignant cells. Due to continued deterioration, his methylprednisolone was increased to 250 mg every 6 hours. Six days after intubation, he had minimal improvement, so salvage therapy with 300 mg of infliximab was initiated. The patient had worsening oxygenation despite mechanical ventilation. Ventilation strategies were also limited due to high peak inspiratory pressures.
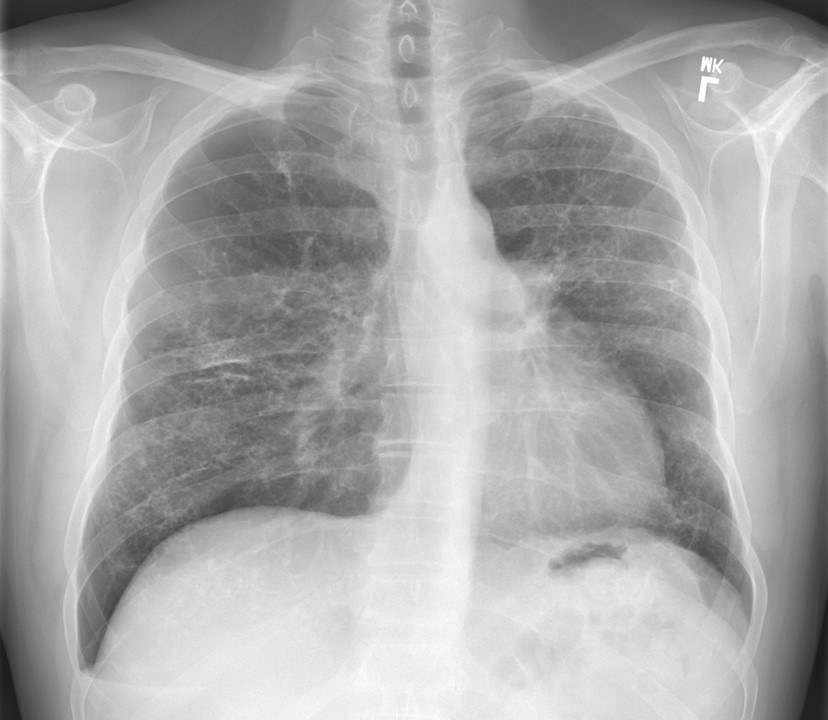
Repeat CT chest without contrast demonstrated worsening extensive interstitial and airspace opacities throughout bilateral lungs (Figure 3).
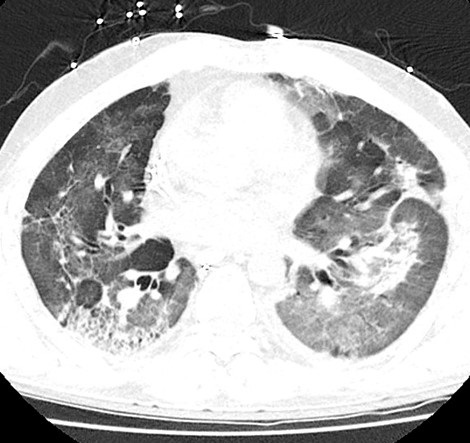 Figure 3. CT chest without contrast after an acute respiratory decompensation demonstrated persistent interstitial and airspace opacities throughout bilateral lungs, significantly worse than the prior CT. To view Figure 3 in a separate, enlarged window click here.
Figure 3. CT chest without contrast after an acute respiratory decompensation demonstrated persistent interstitial and airspace opacities throughout bilateral lungs, significantly worse than the prior CT. To view Figure 3 in a separate, enlarged window click here.
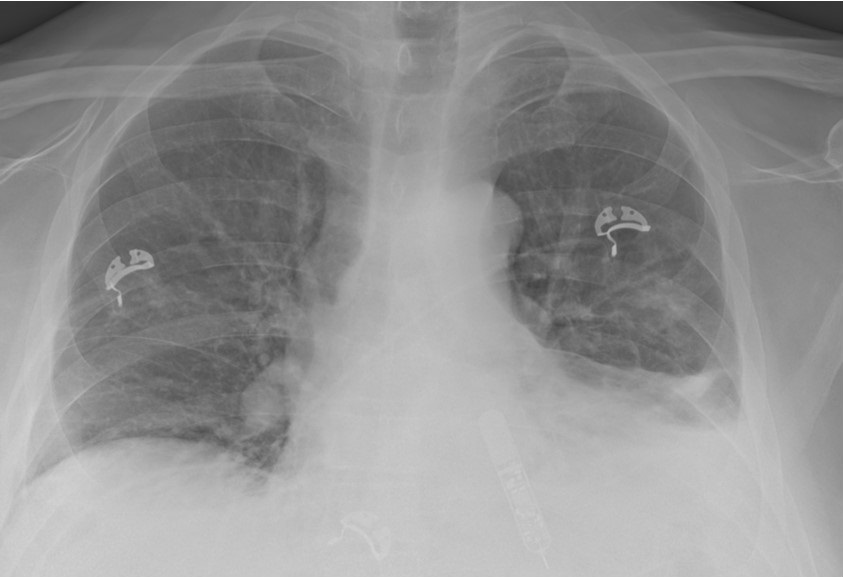
With minimal improvement in his respiratory failure, the patient was transferred to a university hospital to initiate inhaled prostacyclin therapy in an attempt to improve oxygenation and ventilation. He was started on inhaled epoprostenol and cisatracurium infusion. Despite these measures, the patient had no improvement in his respiratory failure with persistent extensive interstitial and airspace opacities throughout bilateral lungs on repeat CT (Figure 4).
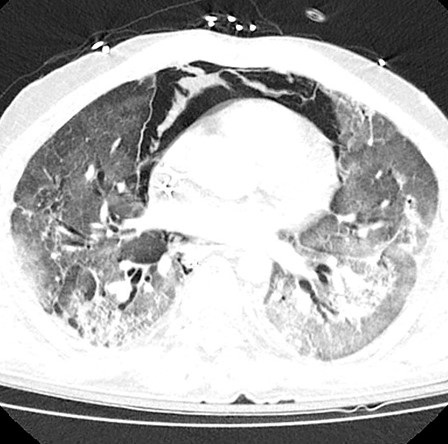 Figure 4. CT pulmonary angiogram showing persistent extensive bilateral ground-glass opacities, scattered consolidative opacities, bronchiectasis, and new pneumomediastinum. To view Figure 4 in a separate, enlarged window click here.
Figure 4. CT pulmonary angiogram showing persistent extensive bilateral ground-glass opacities, scattered consolidative opacities, bronchiectasis, and new pneumomediastinum. To view Figure 4 in a separate, enlarged window click here.
Extracorporeal membrane oxygenation (ECMO) was considered, but he was deemed not an appropriate candidate given the irreversible lung injury. With all avenues for recovery exhausted, the poor prognosis was discussed with the family, who decided to transition to comfort-only care. He expired shortly after cessation of aggressive life support measures.
Discussion
Bleomycin-induced lung injury (BILI) is thought to be due to the development of pulmonary fibrosis, characterized by enhanced production and deposition of collagen and other matrix components (1). Pulmonary toxicity is dose-dependent, with most of these injuries occurring with doses above 400,000 IU. Other risk factors include kidney dysfunction, older age, supplemental oxygen exposure, bolus delivery of infusion, extent of lung metastases, and established lung disease (13). Symptoms and signs include nonproductive cough, dyspnea, pleuritic or substernal chest pain, fever, tachypnea, crackles, lung restriction, and hypoxemia. Clinical manifestations usually develop indolently between one and six months after treatment initiation, but they may persist more than six months after treatment discontinuation. The earliest manifestation of BILI is dyspnea with a reduction in the DLCO (14-15). Best-practice clinical guidelines and the U.S. Food and Drug Administration (FDA) recommend PFTs at baseline and monthly or after each new treatment cycle (16). A DLCO reduction of more than 30-35% should prompt providers to discontinue bleomycin, even if asymptomatic, due to the concern of BILI. However, a recent randomized phase III trial demonstrated that the presence of cough had a higher association with BILI than PFT changes, questioning the benefit of routine PFTs (17).
BILI treatment involves prompt discontinuation of all chemotherapeutic agents. Corticosteroids are given to patients with symptomatic lung toxicity. The suggested prednisone dosing is 0.75 to 1 mg/kg (based on ideal body weight) per day, to a maximum of 100 mg/day, for the first four to six weeks based on clinical data and case reports (3-7). Clinical and radiographic improvement varies by report from 7 to 12 days after early initiation of high-dose corticosteroid therapy (6). There have been two fatal cases of BILI that were attributed to insufficient corticosteroid doses (18). This emphasizes the need for a higher dose to treat the condition effectively. Most patients respond after treatment with limited case reports discussing corticosteroid refractory BILI. These cases have led to the evaluation of off-label therapies as potential treatments. Recent case reports have described imatinib, infliximab, and pirfenidone to have variable success in treating BILI, including those cases refractory to corticosteroids; however, these require long treatment durations for clinical success (8-12).
Etoposide and bleomycin can both cause lung injury; however, the two drugs' mechanisms of injury and clinical presentations can differ (19). Etoposide-induced lung injury typically presents as acute respiratory distress syndrome (ARDS) within hours to days of exposure. In contrast, BILI typically presents as a more gradual onset of pulmonary fibrosis, which can occur weeks to months after exposure. Our patient's clinical course was indolent after discontinuing his chemotherapeutics which is more consistent with a BILI presentation. However, it is difficult to say with the utmost certainty that our patient’s lung injury was not worsened by etoposide.
We present an unusual case of corticosteroid refractory BILI in a young patient with minimal tobacco history and no end-organ dysfunctions. Given the enormous respiratory reserve, most young, healthy patients will develop symptoms only after a severe reduction in diffusion. Our patient did not have the recommended interval PFT monitoring described by clinical guidelines and the FDA. This highlights the importance of interval monitoring, including symptomatic tracking, especially in young patients, in the hopes of early diagnosis of BILI. As this disease usually progresses indolently, monthly PFTs can capture the subtle advancement of lung injury in younger patients. It is uncertain when the initial lung injury began in our patient due to a lack of PFT monitoring during the four treatment cycles of bleomycin. However, if changes had been detected, earlier management could have been implemented, such as earlier discontinuation of chemotherapy, increasing corticosteroids, or off-label therapies.
This case also accentuates the limited data on off-label treatment options for corticosteroid refractory BILI. Our patient developed progressive pulmonary fibrosis, ultimately leading to his demise. Although he received infliximab as salvage therapy, it is improbable that this treatment would have had benefit due to the late fibrosing stage of his disease presentation. Universally, an immunomodulatory agent’s efficacy wanes dramatically once in the terminal fibrosing stages of many interstitial lung diseases, reiterating the need for early diagnosis and aggressive treatment during the inflammatory phase (20). If our patient had been identified sooner as refractory to corticosteroids, prompter introduction of second-line agents might have resulted in an alternative clinical outcome. Maximizing medical management in this patient population is particularly critical given that other salvage treatments like ECMO and lung transplantation are not recommended and are usually contraindicated. Additional prospective investigation in refractory disease is necessary to better validate and quantify the therapeutic efficacy of available second-line and off-line medical therapies.
Conclusion
Patients on bleomycin therapy are at risk of developing BILI associated dyspnea that may present as progressive pulmonary fibrosis, hypersensitivity pneumonitis, or organizing pneumonia. If a patient treated with bleomycin continues to have unremitting shortness of breath, the concern for BILI should be high and may warrant earlier evaluation and intervention.
Acknowledgments
Christopher S Dossett, Kelli Kosako Yost, Christopher Lau, and Nafis Shamsid-Deen contributed to the drafting and revising of this manuscript. The authors have no conflict of interest. All authors have consented to the approval of this manuscript.
References
- Reinert T, Baldotto C, Nunes F, Scheliga A. Bleomycin-Induced Lung Injury. Journal of Cancer Research 2013;2013:1-9. [CrossRef]
- Hay J, Shahzeidi S, Laurent G. Mechanisms of bleomycin-induced lung damage. Arch Toxicol. 1991;65(2):81-94. [CrossRef] [PubMed]
- White DA, Stover DE. Severe bleomycin-induced pneumonitis. Clinical features and response to corticosteroids. Chest. 1984 Nov;86(5):723-8. [CrossRef] [PubMed]
- Ghalamkari M, Khatuni M, Toogeh G, Haghighi S, Taherkhani M. Reversible Acute Lung Injury due to Bleomycin. Tanaffos. 2022 Feb;21(2):253-256. [PubMed]
- Rashid RS. Bleomycin lung: a case report. BMJ Case Rep. 2009;2009:bcr11.2008.1175. [CrossRef] [PubMed]
- Gupta R, Ettinger NA. Beyond conventional therapy: role of pulse steroids in bleomycin induced lung injury. Respir Care. 2014 Jan;59(1):e9-e12. [CrossRef] [PubMed]
- Wang, X, Deng, J, Sothwal, A, Gordon, E, Patel, G. Bleomycin-Induced Pneumonitis Responds To Super-High-Dose Steroid and Monitored By LDH and PAO2/FIO2. Critical Care Medicine 2016;44(12):558. [CrossRef]
- Banakh I, Lam A, Tiruvoipati R, Carney I, Botha J. Imatinib for bleomycin induced pulmonary toxicity: a case report and evidence-base review. Clin Case Rep. 2016 Apr 1;4(5):486-90. [CrossRef] [PubMed]
- Ge V, Banakh I, Tiruvoipati R, Haji K. Bleomycin-induced pulmonary toxicity and treatment with infliximab: A case report. Clin Case Rep. 2018 Sep 4;6(10):2011-2014. [CrossRef] [PubMed]
- Carnevale-Schianca F, Gallo S, Rota-Scalabrini D, Sangiolo D, Fizzotti M, Caravelli D, Capaldi A, Anselmetti G, Palesandro E, D'Ambrosio L, Coha V, Obert R, Aglietta M, Grignani G. Complete resolution of life-threatening bleomycin-induced pneumonitis after treatment with imatinib mesylate in a patient with Hodgkin's lymphoma: hope for severe chemotherapy-induced toxicity? J Clin Oncol. 2011 Aug 20;29(24):e691-3. [CrossRef] [PubMed]
- Aykaç N, Tecimer C. Imatinib Treatment for Bleomycin-Induced Pulmonary Toxicity. Turk Thorac J. 2020 Nov;21(6):457-460. [CrossRef] [PubMed]
- Sakamoto K, Ito S, Hashimoto N, Hasegawa Y. Pirfenidone as salvage treatment for refractory bleomycin-induced lung injury: a case report of seminoma. BMC Cancer. 2017 Aug 7;17(1):526. [CrossRef] [PubMed]
- Comis RL. Bleomycin pulmonary toxicity: current status and future directions. Semin Oncol. 1992 Apr;19(2 Suppl 5):64-70. [PubMed]
- Lucraft HH, Wilkinson PM, Stretton TB, Read G. Role of pulmonary function tests in the prevention of bleomycin pulmonary toxicity during chemotherapy for metastatic testicular teratoma. Eur J Cancer Clin Oncol. 1982 Feb;18(2):133-9. [CrossRef] [PubMed]
- Nippon Kayaku Co., Ltd. Blenoxane (bleomycin sulfate) [package insert]. U.S. Food and Drug Administration website. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/050443s036lbl.pdf. Revised April 2010. Accessed April 15, 2023.
- Watson RA, De La Peña H, et al. Development of a best-practice clinical guideline for the use of bleomycin in the treatment of germ cell tumours in the UK. Br J Cancer. 2018 Oct;119(9):1044-1051. [CrossRef] [PubMed]
- Shamash J, Sarker SJ, Huddart R, et al. A randomized phase III study of 72 h infusional versus bolus bleomycin in BEP (bleomycin, etoposide and cisplatin) chemotherapy to treat IGCCCG good prognosis metastatic germ cell tumours (TE-3). Ann Oncol. 2017 Jun 1;28(6):1333-1338. [CrossRef] [PubMed]
- Bloor AJ, Seale JR, Marcus RE. Two cases of fatal bleomycin pneumonitis complicating the treatment of non-Hodgkin's lymphoma. Clin Lab Haematol. 1998 Apr;20(2):119-21. [CrossRef] [PubMed]
- Gurjal A, An T, Valdivieso M, Kalemkerian GP. Etoposide-induced pulmonary toxicity. Lung Cancer. 1999 Nov;26(2):109-12. [CrossRef] [PubMed]
- Davies HR, Richeldi L, Walters EH. Immunomodulatory agents for idiopathic pulmonary fibrosis. Cochrane Database Syst Rev. 2003;(3):CD003134. [CrossRef] [PubMed]
Cite as: Dossett CS, Yost KK, Lau C, Shamsid-Deen N. A case of progressive bleomycin lung toxicity refractory to steroid therapy. Southwest J Pulm Crit Care Sleep. 2023;26(6):90-96. doi:
https://doi.org/10.13175/swjpccs013-23 PDF
 Friday, March 1, 2024 at 8:00AM
Friday, March 1, 2024 at 8:00AM  Figure 1. Selected images from thoracic CT done November 2023 showing RLL mass (A, red arrow) and LLL mass (B, blue arrow).
Figure 1. Selected images from thoracic CT done November 2023 showing RLL mass (A, red arrow) and LLL mass (B, blue arrow).