Impact of Cytomegalovirus DNAemia Below the Lower Limit of Quantification: Multistate Model in Lung Transplant Recipients
 Thursday, November 17, 2022 at 8:00AM
Thursday, November 17, 2022 at 8:00AM Spenser E. January, PharmD1
Daniel Britt, PharmD1
April A. Pottebaum, PharmD1
Tamara T. Krekel, PharmD1
Ramsey R. Hachem, MD2
Rodrigo Vazquez-Guillamet, MD2
1Department of Pharmacy, Barnes-Jewish Hospital, Saint Louis, MO, USA
2Division of Pulmonary and Critical Care, Washington University in Saint Louis, Saint Louis, MO, USA
Abstract
Background
Cytomegalovirus (CMV) infections following transplantation lead to significant morbidity. Identification of CMV DNAemia continually improves and now viral loads below the lower limit of quantification (LLOQ) are detectable. However, the clinical course of positive CMV qPCR <LLOQ is unknown.
Methods
This retrospective study included lung transplant recipients experiencing their first episode of positive CMV qPCR <LLOQ with all qPCR assays conducted using COBAS® AmpliPrep/COBAS® TaqMan®. A Markov-like multistate model was utilized to describe the course of CMV DNAemia <LLOQ. A multivariable model was employed to identify predictors of transitioning to a positive, quantifiable state.
Results
100 patients with a CMV <LLOQ result were included, encompassing 1,248 transitions in the six months following first episode of CMV qPCR <LLOQ. There was an 97.8% probability of remaining <LLOQ or undetectable and a 2.2% probability of transitioning to a positive, quantifiable qPCR state. The multivariable regression model identified treatment for rejection, increasing body mass index, valganciclovir therapy, and increasing CMV qPCR viral load as being predictive of transitioning from CMV qPCR undetectable or <LLOQ into a positive, quantifiable CMV qPCR state.
Conclusions
Most patients did not transition into a positive, quantifiable CMV qPCR state following a first episode of positive CMV qPCR <LLOQ in this cohort of lung transplant recipients.
Article Abbreviations:
- <LLOQ: below the lower limit of quantification
- BMI: body mass index; CMV: cytomegalovirus
- IgG: immunoglobulin G
- IUnit: international units
- qPCR: quantitative polymerase chain reaction
Introduction
Even with recent advancements in its diagnosis and treatment, cytomegalovirus (CMV) infection continues to be one of the most common complications after transplantation causing significant morbidity and contributing to mortality. Lung transplant recipients are especially afflicted; shorter telomere length has been associated with increased risk of CMV DNAemia, especially in patients with idiopathic pulmonary fibrosis (1,2). Compared to other solid organ transplants, previously uninfected lung recipients have the highest rate of CMV donor derived infections (3). Once infected with CMV, patients are at risk of disease related to virus replication but also to adverse outcomes via indirect pathways leading to acute or chronic rejection and superinfections with other pathogens (4).
To prevent these adverse outcomes, the current standard of care is to implement primary prevention strategies including universal prophylaxis and pre-emptive therapy. In the universal prophylaxis
strategy, every patient at high risk for CMV (e.g., recipient previously unexposed to CMV [CMV seronegative] receiving an organ from a CMV seropositive donor) is treated with valganciclovir, an oral antiviral highly active against CMV, for a period varying from 3 months to 1 year, followed by continued monitoring. In pre-emptive therapy, CMV quantitative polymerase chain reaction (qPCR) in whole blood or plasma is performed every week and active treatment is initiated if a pre-specified level of quantification is reached. This threshold is program and test dependent due to continued variability in reagents, amplification, and extraction techniques (5,6).
Contemporary assays for the quantification of CMV in the blood are increasingly sensitive. Previous solid organ transplant studies have attempted to establish a threshold viral load to initiate treatment, which has ranged from a plasma cutoff of 1,500 IUnit/mL (7-10) to 5,087 copies/mL (11) and a whole blood cutoff of 800 copies/mL (12), but these studies did not include lung transplant recipients (7-10,12), did not include patients over one year from transplant (7-11), did not include all CMV serostatus groups (7,8,10) or were performed prior to the development of highly sensitive CMV qPCR assays (11). As a result, there is no consensus on when to initiate treatment in lung transplant recipients with asymptomatic DNAemia. Most experts will treat asymptomatic DNAemia with antivirals with the intent of preventing CMV related morbidity. However, with ever-increasing sensitivities of the assays, the probability of detection of clinically insignificant levels of DNAemia increases along with the risk of unnecessary exposure to treatment toxicities to the patient.
CMV remains in the host for life in one of three possible states: 1. CMV latent infection, when it can’t be detected in blood 2. CMV infection, when it is detected in blood but there are no symptoms of disease, and 3. CMV disease when signs of infection and clinical disease are present. Since CMV remains in the host, outcomes cannot be thought of as dichotomously cured or not cured; thus, the decision to treat or observe should consider the probabilities of transitioning between these states. We evaluated the probability of disease progression and spontaneous resolution for patients with CMV qPCR levels below the lower limit of quantification (<LLOQ). We hypothesize that a great majority of patients with positive qPCR <LLOQ will not progress to CMV infection or disease without treatment and that treatment might only be warranted in patients at especially high risk for progression.
Materials and Methods
This was a single-center retrospective cohort study conducted at Barnes-Jewish Hospital and Washington University in Saint Louis, Missouri. At our institution, the CMV qPCR assay in use since April 1, 2017 is the COBAS® AmpliPrep/COBAS® TaqMan® CMV assay which uses plasma specimens and had a quantifiable limit of ≥137 IUnits/mL during the study period. Adult lung transplant recipients were included in this study if they were transplanted between April 1, 2009 and April 1, 2019 and had a plasma CMV qPCR sample with CMV detected but <LLOQ (<137 IUnits/mL) between April 1, 2017 and July 1, 2019. All patients were followed for a duration of six months following their CMV <LLOQ result. Patients were excluded if they obtained any CMV qPCR during the study period at an outside laboratory, if they had no subsequent CMV qPCR or routine laboratory monitoring performed in the six months following the episode of positive CMV qPCR <LLOQ, if they had an episode of quantifiable CMV DNAemia without clearance (defined as two consecutive undetectable CMV qPCR results) prior to the positive CMV qPCR <LLOQ result, or if they were on valganciclovir at the time of CMV qPCR <LLOQ result. We did not consider CMV bronchoalveolar lavage samples as tissue invasive disease and they were not included in the analysis. At Barnes-Jewish Hospital, the institutional protocol for lung transplant recipients is to administer valganciclovir prophylaxis for six months after transplant (followed by acyclovir for life) if the donor is CMV IgG seropositive and the recipient is CMV IgG seronegative; if the patient is CMV IgG seropositive or both donor and recipient are CMV IgG seronegative at the time of transplant they are given acyclovir for life. CMV qPCRs are monitored weekly during the first 3 months after transplant, then monthly for the remainder of the first year. The decision to initiate a CMV-active antiviral following a result of positive CMV qPCR <LLOQ is at the discretion of the treating physician; there is no institutional standard viral load at which to initiate antiviral therapy. CMV IVIg is not routinely used for prophylaxis or treatment at our institution. Induction immunosuppression for lung transplant recipients at Barnes-Jewish Hospital is typically intra-operative methylprednisolone 500 mg in conjunction with basiliximab 20 mg on post-operative day 0 and post-operative day 4; maintenance immunosuppression includes tacrolimus with a goal trough of 7-10 ng/mL for the first post-transplant year and 4-7 ng/mL thereafter, mycophenolate mofetil 1,000 mg twice daily, and prednisone tapered to 5 mg daily by six months post-transplant. In the instance of a CMV qPCR <LLOQ result, institutional practice is to not decrease maintenance immunosuppression unless another compelling indication to do so is present (e.g., leukopenia). If maintenance immunosuppression was decreased, the rationale behind the decrease was collected.
Statistical Analysis
Continuous data are presented as mean and standard deviations and compared using Student’s T test, categorical data as percentages and compared using Chi square or Fisher’s exact test as appropriate.
Markov like model: In Markov analysis, individuals can be in one of several “states”. The unit of analysis is a transition and not individuals. A transition is defined as moving from one state to another or staying in the same state. Transitions are further classified as favorable if the patient remained in the healthy <LLOQ state, transitioned from CMV infection to healthy <LLOQ or from CMV disease to CMV infection or healthy <LLOQ state. All other transitions were considered adverse. An advantage of this type of analysis is the possibility of providing patients and physicians not only with the probability of progression of their disease but also the probability of getting better and reversion to a healthy state. Markov analysis has been previously utilized to evaluate bacteremia states in patients with sepsis.13 To perform the analysis, all patients need to be evaluated at fixed time intervals, referred to as the Markov cycle. The mean interval between CMV testing was 13 days with median and interquartile range of 7 (7-14), based on these information and previous knowledge of response time for CMV viral load the Markov cycle was set at 14 days. Like most medical processes, transitions were not completely independent of the previous state so the strict definition of Markov modeling is unmet; therefore, a Markov-like model was implemented for transition analysis. Since the use of Markov modeling performed in this study is purely descriptive, and we did not project it into the future over multiple cycles, this is still appropriate.
Markov-model states: a priori we considered three states. A healthy state LLOQ was defined if the patient had either unquantifiable CMV or <LLOQ and no evidence of tissue invasive CMV disease. CMV infection was defined as CMV above LLOQ but without clinical symptoms or signs of invasive disease and lastly, CMV disease was defined as CMV quantifiable in blood and symptoms (fever for >2 days or malaise) or tissue invasive disease was present.
Patients lost to follow up before the six-month mark were censored at that point. Missing qPCRs were imputed as the mean from the previous and posterior state, when more than one consecutive qPCR was missing the transition was coded as missing. Transition probabilities are presented as percentages.
Multivariable logistic regression with adverse transition as an outcome was performed; factors considered for inclusion into the model were based on variables associated with progression of CMV DNAemia on univariable analysis at a P value of <0.10 and physiological plausibility to affect progression. Predictors were transition specific (e.g., valganciclovir, immunosuppression changes were accounted for only if given in the prior state). SPSS version 25 and Stata SE 15.1 (Stata-Corp, LLC) were used for statistical analysis with significance defined as a P value ≤ 0.05. This study was approved by the institutional review board with a waiver of consent given the retrospective nature of the study.
Results
Cohort characteristics
A total of 100 lung transplant recipients met inclusion criteria (Figure 1).
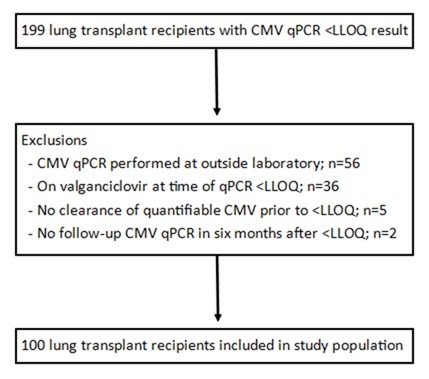
Figure 1. Study enrollment. To view an Figure 1 in an enlarged, separate window click here.
There were 50 (50%) females, recipients were mostly white and 86% of patients were on three-drug maintenance immunosuppression at the time of CMV PCR <LLOQ episode (mainly tacrolimus, mycophenolate mofetil, and prednisone); only one patient was maintained on a mechanistic target of rapamycin inhibitor. For induction, 95% received basiliximab in addition to methylprednisolone, two patients received equine antithymocyte globulin, two patients received rabbit antithymocyte globulin, and one patient received monotherapy induction with methylprednisolone. Seventy-three patients had their CMV qPCR <LLOQ within the first post-transplant year. Baseline characteristics are detailed in Table 1.
Table 1. Baseline characteristics.
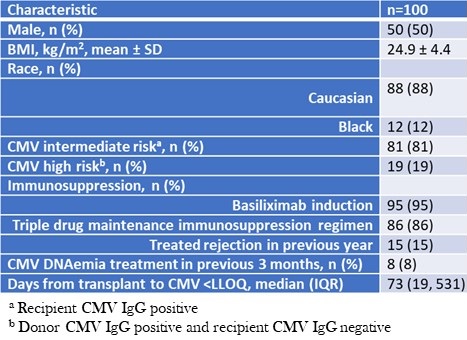
To view Table 1 in an enlarged, separate window click here.
None of the patients who had a positive CMV qPCR <LLOQ episode were seronegative recipients of seronegative donors. Following the initial positive CMV qPCR <LLOQ, CMV qPCR was rechecked a median of 10 times per patient in the six months of follow-up. The median time from transplant to first positive qPCR was 73 (interquartile range 19 - 531) days. The median peak DNAemia was 535 (interquartile range 243 – 1249) IUnit/mL. Following the first episode of CMV PCR <LLOQ, 70 patients were monitored, 9 patients were placed on prophylactic antiviral dosing, and 21 patients were placed on treatment antiviral dosing. Valganciclovir was ultimately prescribed in 64 patients (four patients were prescribed ganciclovir before transitioning to valganciclovir). Thirty-two patients received intravenous immunoglobulins in the six-month follow-up period (none received CMV IVIg), mainly for donor specific antibodies or hypogammaglobulinemia. No patient required the use of cidofovir or foscarnet, and there were no cases of ganciclovir resistance.
During the follow up period, maintenance immunosuppression was de-escalated in 25% of patients. Of the 25 patients classified as de-escalation, all were related to antimetabolite being dose reduced, held, or both. The most common cause for de-escalation was leukopenia (56%), followed by CMV infection itself (24%), and two patients each had their antimetabolite held for diarrhea and cancer. Maintenance immunosuppression was intensified in 25% of patients. Intensification of immunosuppression occurred due to treatment for rejection, donor-specific antibodies, chronic lung allograft dysfunction, or a combination thereof. Therapies received included rabbit antithymocyte globulin (n=10), rituximab (n=6), methylprednisolone (n=3), carfilzomib (n=2), cyclophosphamide (n=1), and tocilizumab (n=1). The antimetabolite (mycophenolate or azathioprine) was resumed in four patients, and one patient was initiated on everolimus.
Transition analysis
The total number of transitions in the six months of follow-up was 1,248. Adverse transitions occurred 76 times (6.1% of all transitions). Of these adverse transitions, 26 (2.1%) were from healthy <LLOQ to CMV infection and 50 (4%) from CMV infection remained in the CMV infection state. Favorable transitions occurred 1174 times; 24 (1.9%) from CMV infection to healthy LLOQ and 1148 (92%) from healthy <LLOQ remained in the healthy <LLOQ state. There were 0 (0%) transitions to or from CMV disease. Transition probabilities between states are depicted in figure 2; from a state of healthy <LLOQ, the probability of remaining in this state on the subsequent Markov cycle (14 days later) was 97.8% and the probability of transitioning into the adverse CMV infection state was 2.2%.
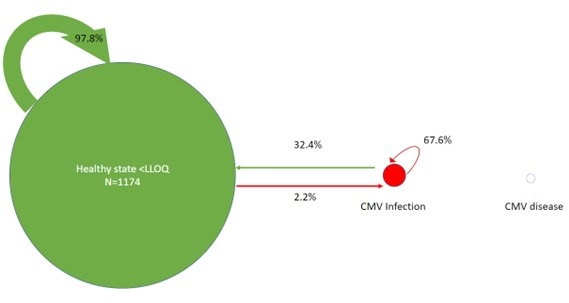
Figure 2. Markov-like model of transition probabilities between healthy state <LLOQ, CMV infection, and CMV disease. The arrow thickness and bubble size are proportional to the number of transitions between states. Percentages reflect the probability of transitioning between states from one Markov cycle to the next. To view Figure 2 in an enlarged, separate window click here.
A multivariable logistic regression analysis was used to identify risk factors for adverse transitions to the CMV infection state. A higher body mass index (BMI), recent intensification of immunosuppression, prescription of valganciclovir (after CMV pPCR <LLOQ result) and having higher CMV qPCR values in subsequent Markov cycles following initial CMV <LLOQ were predictive of transitioning into, or staying in, the CMV infection state (Table 2).
Table 2. Multivariable regression analysis of predictors adverse transitions: transitioning into, or staying in the state of, positive, quantifiable CMV DNAemia.
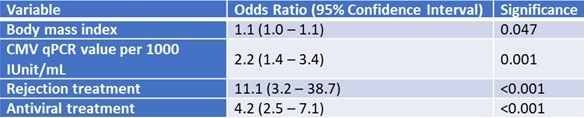
To view Table 2 in an enlarged, separate window click here.
To understand the increased risk of detrimental transitions while on treatment with valganciclovir we performed a logistic regression model with treatment with valganciclovir as the outcome. Induction with thymoglobulin (OR= 4.4; 95%CI: 2.6 , 7.6; p<0.001), transplant from a CMV seropositive donor to a seronegative recipient (OR= 2.5; 1.9, 3.3; p<0.001), number of immunosuppressive drugs (OR=1.1; 95% CI: 1.03, 1.16; p=0.002) were predictors in this model. Additionally, intensification of treatment with rabbit antithymocyte globulin was a perfect predictor of treatment with valganciclovir (10/10).
Discussion
Although previous solid organ transplant studies have sought to define a CMV viral load at which to initiate antiviral therapy, there remains no consensus on this threshold which is likely due to the heterogeneity in transplant populations studied and inter-assay variability (6-11, 14). Since no definitive quantity of CMV detected in the bloodstream has been established, this study evaluated the probability of transitioning from an undetectable or <LLOQ qPCR for CMV into a state of positive, quantifiable viral load (CMV infection) in lung transplant recipients to guide the use of antiviral treatment. Our results support a monitoring strategy in patients with CMV qPCR < LLOQ given the small probability of transitioning from qPCR <LLOQ to CMV infection and lack of further transition into CMV disease, which occurred in no patients. It should be noted that since routine invasive testing (e.g., biopsy of the GI tract) is not performed without cause at our institution, it is possible that patients could have had undiagnosed tissue invasive CMV. Valganciclovir is an effective treatment for CMV but has associated side effects such as leukopenia and neutropenia which have been reported in 13.5% and 8.2% of transplant patients, respectively (15). In the solid organ transplant population, these hematological abnormalities may be already present or exacerbated by baseline immunosuppression or short telomeres specifically in the lung transplant population (1). It is important to establish the patient population where the benefit of antiviral treatment outweighs the potential additive medication toxicities of valganciclovir when a result of positive CMV qPCR <LLOQ is encountered. Initiation of treatment might be warranted in patients undergoing intensification of immunosuppression and special attention should be given to patients with higher BMI as these were predictive of transitioning to CMV infection in the multivariable adjustment model.
The role of immunosuppression in the development of CMV infection and disease is well known, with risk factors for CMV including donor positive/recipient negative serostatus, short prophylaxis courses following transplantation, higher intensity immunosuppression, and rejection (5). The multivariable model in this study identified higher intensity immunosuppression as being an independent risk factor for progression from CMV qPCR <LLOQ to CMV infection. This study also found BMI to be a predictor of adverse transitions. Based on previous evidence, BMI is likely to be functioning as a proxy for the presence of metabolic syndrome. In the general population, the presence of metabolic syndrome independent of obesity is a risk factor for continued shedding of CMV in urine of infected individuals as well as for higher levels of DNAemia (16,17). Alternatively, patients with higher BMIs may be relatively underdosed when treated using fixed-dose oral valganciclovir.
Thirty percent of patients were placed on valganciclovir following their first episode of CMV <LLOQ; however, ultimately, 64% of this cohort was placed on valganciclovir during the six months of follow-up. The non-uniform time of valganciclovir initiation is a limitation of the current study. In the transitional analysis, treatment with valganciclovir was strongly associated with detrimental transitions (e.g., going from <LLOQ to CMV infection or remaining in CMV infection state). This finding should be understood as an association and not causation. Risk of treatment with valganciclovir was predicted by enhanced immunosuppression and belonging to the high-risk group of seropositive donor to seronegative recipient, suggesting confounding by prognosis. Patients at higher risk of progression and patients with DNAemia were more likely to be on treatment. Patients remaining in CMV infection are more likely to be on treatment with valganciclovir. The effectiveness of oral valganciclovir for the treatment of CMV DNAemia has been clearly demonstrated in randomized clinical trials and retrospective cohorts. In clinical practice, physicians are more likely to initiate treatment for <LLOQ CMV qPCR in patients they think are at a high risk of progressing to CMV infection or disease. For example, there likely is a lower threshold to initiate treatment for <LLOQ CMV in patients who are going to be treated for acute rejection. In this case valganciclovir treatment may reflect the perceived risk of CMV infection or disease by the treating clinician.
This study is limited by its retrospective nature; the duration and frequency of CMV qPCR monitoring were not uniform once patients were discharged from the hospital, use of CMV-active antivirals was determined via review of the electronic health record, and variables potentially associated with transitioning to a positive, quantifiable CMV DNAemia state could not be exhaustively evaluated. Large changes of immunosuppression, such as starting or stopping an immunosuppressant, was captured but smaller changes such as changes in tacrolimus goal troughs were not assessable. Additionally, our center utilizes a pre-emptive strategy for intermediate risk CMV patients (recipient CMV IgG positive) which differs from guideline recommendations and may limit generalizability to other institutions.18 It is unknown whether CMV <LLOQ and an undetectable CMV PCR result have the same impact on long-term allograft outcomes. In order to rigorously evaluate CMV PCR values it was necessary to restrict to one specific assay given inter-assay variability; this could have introduced bias by excluding patients who do not follow as closely at Barnes-Jewish Hospital. At Barnes-Jewish Hospital, lung transplant patients are placed on a CMV-active antiviral following a result of positive CMV qPCR <LLOQ at the discretion of the treating physician based on a variety of patient-specific factors. Since there is no protocol on how to manage an episode of positive CMV qPCR <LLOQ, selection bias played a role in the treatment of the patients in this cohort. Basiliximab is the induction agent of choice at Barnes-Jewish Hospital, which may limit the generalizability of these results for patients treated with antithymocyte globulin at other centers. Six months was chosen as the follow-up period to isolate the effect of chosen study variables on transitioning out of CMV undetectable or <LLOQ state. Longer studies may be helpful to determine the effects of a positive CMV qPCR <LLOQ episode that may extend past six months, and prospective studies will be beneficial to isolate and fully elucidate the effect of antiviral therapy on the course of CMV following a positive qPCR <LLOQ result.
In conclusion, there was a low risk of transitioning to a higher CMV viral load following an initial CMV qPCR result of <LLOQ, and risk factors for progression include intensification of immunosuppression (such as treatment for rejection) and higher BMI. Higher CMV viral loads were also associated with an increased risk of transitioning back into or staying in the state of positive, quantifiable CMV DNAemia. In the absence of the specific risk factors, it may be reasonable to serially monitor CMV qPCR as opposed to initiating antiviral therapy which may lead to toxicity. Larger, prospective studies are needed to fully determine the effect of CMV-active antivirals on low level CMV DNAemia.
Acknowledgements
The authors wish to acknowledge Karen Bennett Bain and Anne Thorndyke for their contributions to study design.
Funding: the authors received no sources of funding for this research.
Disclosures: none
References
- Courtwright AM, Lamattina AM, Takahashi M, et al. Shorter telomere length following lung transplantation is associated with clinically significant leukopenia and decreased chronic lung allograft dysfunction-free survival. ERJ Open Res. 2020 Jun 15;6(2):00003-2020. [CrossRef] [PubMed]
- Popescu I, Mannem H, Winters SA, et al. Impaired Cytomegalovirus Immunity in Idiopathic Pulmonary Fibrosis Lung Transplant Recipients with Short Telomeres. Am J Respir Crit Care Med. 2019 Feb 1;199(3):362-376. [CrossRef] [PubMed]
- Duncan AJ, Dummer JS, Paradis IL, et al. Cytomegalovirus infection and survival in lung transplant recipients. J Heart Lung Transplant. 1991 Sep-Oct;10(5 Pt 1):638-44; discussion 645-6. [PubMed]
- Bando K, Paradis IL, Komatsu K, et al. Analysis of time-dependent risks for infection, rejection, and death after pulmonary transplantation. J Thorac Cardiovasc Surg. 1995 Jan;109(1):49-57; discussion 57-9. [CrossRef] [PubMed]
- Kotton CN, Kumar D, Caliendo AM, Huprikar S, Chou S, Danziger-Isakov L, Humar A; The Transplantation Society International CMV Consensus Group. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation. Transplantation. 2018 Jun;102(6):900-931. [CrossRef] [PubMed]
- Preiksaitis JK, Hayden RT, Tong Y, et al. Are We There Yet? Impact of the First International Standard for Cytomegalovirus DNA on the Harmonization of Results Reported on Plasma Samples. Clin Infect Dis. 2016 Sep 1;63(5):583-9. [CrossRef] [PubMed]
- Martin-Gandul C, Perez-Romero P, Sanchez M, et al. Determination, validation and standardization of a CMV DNA cut-off value in plasma for preemptive treatment of CMV infection in solid organ transplant recipients at lower risk for CMV infection. J Clin Virol. 2013 Jan;56(1):13-8. [CrossRef] [PubMed]
- Martin-Gandul C, Perez-Romero P, Blanco-Lobo P, et al. Viral load, CMV-specific T-cell immune response and cytomegalovirus disease in solid organ transplant recipients at higher risk for cytomegalovirus infection during preemptive therapy. Transpl Int. 2014 Oct;27(10):1060-8. [CrossRef] [PubMed]
- Boaretti M, Sorrentino A, Zantedeschi C, Forni A, Boschiero L, Fontana R. Quantification of cytomegalovirus DNA by a fully automated real-time PCR for early diagnosis and monitoring of active viral infection in solid organ transplant recipients. J Clin Virol. 2013 Feb;56(2):124-8. [CrossRef] [PubMed]
- David-Neto E, Triboni AHK, Paula FJ, et al. A double-blinded, prospective study to define antigenemia and quantitative real-time polymerase chain reaction cutoffs to start preemptive therapy in low-risk, seropositive, renal transplanted recipients. Transplantation. 2014 Nov 27;98(10):1077-81. [CrossRef] [PubMed]
- Weinberg A, Hodges TN, Li S, Cai G, Zamora MR. Comparison of PCR, antigenemia assay, and rapid blood culture for detection and prevention of cytomegalovirus disease after lung transplantation. J Clin Microbiol. 2000 Feb;38(2):768-72. [CrossRef] [PubMed]
- Madi N, Al-Nakib W, Mustafa AS, Saeed T, Pacsa A, Nampoory MR. Detection and monitoring of cytomegalovirus infection in renal transplant patients by quantitative real-time PCR. Med Princ Pract. 2007;16(4):268-73. [CrossRef] [PubMed]
- Guillamet MCV, Vazquez R, Noe J, Micek ST, Fraser VJ, Kollef MH. Impact of Baseline Characteristics on Future Episodes of Bloodstream Infections: Multistate Model in Septic Patients With Bloodstream Infections. Clin Infect Dis. 2020 Dec 15;71(12):3103-3109. [CrossRef] [PubMed]
- Natori Y, Alghamdi A, Tazari M, et al. Use of Viral Load as a Surrogate Marker in Clinical Studies of Cytomegalovirus in Solid Organ Transplantation: A Systematic Review and Meta-analysis. Clin Infect Dis. 2018 Feb 1;66(4):617-631. [CrossRef] [PubMed]
- Paya C, Humar A, Dominguez E, et al. Efficacy and safety of valganciclovir vs. oral ganciclovir for prevention of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant. 2004 Apr;4(4):611-20. [CrossRef] [PubMed]
- Fleck-Derderian S, McClellan W, Wojcicki JM. The association between cytomegalovirus infection, obesity, and metabolic syndrome in U.S. adult females. Obesity (Silver Spring). 2017 Mar;25(3):626-633. [CrossRef] [PubMed]
- Hamer M, Batty GD, Kivimäki M. Obesity, Metabolic Health, and History of Cytomegalovirus Infection in the General Population. J Clin Endocrinol Metab. 2016 Apr;101(4):1680-5. [CrossRef] [PubMed]
- Razonable RR, Humar A. Cytomegalovirus in solid organ transplant recipients-Guidelines of the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019 Sep;33(9):e13512. [CrossRef] [PubMed]
Cite as: January SE, Britt D, Pottebaum AA, Krekel TT, Hachem RR, Vazquez-Guillamet R. Impact of Cytomegalovirus DNAemia Below the Lower Limit of Quantification: Multistate Model in Lung Transplant Recipients. Southwest J Pulm, Crit Care Sleep. 2022;25(5):73-82. doi: https://doi.org/10.13175/swjpccs045-22 PDF








