Long-term All-Cause Mortality Risk in Obstructive Sleep Apnea Using Hypopneas Defined by a ≥3 Percent Oxygen Desaturation or Arousal
 Monday, July 12, 2021 at 8:00AM
Monday, July 12, 2021 at 8:00AM Rohit Budhiraja, MD1
Stuart F. Quan, MD1,2
1Division of Sleep and Circadian Disorders, Brigham and Women’s Hospital, Boston, MA
2Arizona Asthma and Airways Research Center, University of Arizona College of Medicine, Tucson, AZ
Abstract
Study Objectives: Some prior studies have demonstrated an increase in mortality associated with obstructive sleep apnea (OSA) utilizing a definition of OSA that requires a minimum 4% oxygen desaturation to identify a hypopnea. No large community-based studies have determined the risk of long-term mortality with OSA with hypopneas defined by a ≥3% O2 desaturation or arousal (AHI3%A).
Methods: Data from 5591 Sleep Heart Health Study participants without prevalent cardiovascular disease at baseline who underwent polysomnography were analyzed regarding OSA diagnosed using the AHI3%A criteria and all-cause mortality over a mean follow up period of 10.9±3.2 years.
Results: There were 1050 deaths in this group during the follow-up period. A Kaplan-Meir plot of survival revealed a reduction in survival with increasing AHI severity. Cox proportional hazards regression models revealed significantly increased all-cause mortality risk with increasing AHI, hazard ratio (HR, 95% CI) 1.13 (1.04-1.23), after adjusting for age, sex, race, BMI, cholesterol, HDL, self-reported hypertension and/or diabetes and smoking status. In categorical models, the mortality risk was significantly higher with severe OSA [adjusted HR 1.38 (1.09-1.76)]. When stratified by gender or age, severe OSA was associated with increased risk of death in men [adjusted HR 1.14 (1.01-1.28)] and in those <70 years of age [adjusted HR 1.51 (1.02-2.26)]. In contrast, AHI severity was not associated with increased mortality in women or those ≥70 years of age in fully adjusted models.
Conclusion: Severe AHI3%A OSA is associated with significantly increased mortality risk, especially in men and those <70 years of age.
Introduction
Obstructive sleep apnea (OSA) is a prevalent disorder associated with diverse physiological changes. Intermittent hypoxia-reoxygenation, sympathetic nervous system activation and endothelial dysfunction have been demonstrated in OSA and likely contribute to adverse outcomes including daytime sleepiness, hypertension, coronary artery disease, and stroke (1,2). It is also associated with increased mortality, especially in those with more severe disease (3-7).
The severity of OSA is most frequently categorized using the apnea hypopnea index (AHI). However, the definition of the ‘hypopnea’ component of this index remains a matter of controversy. American Academy of Sleep Medicine (AASM) guidelines recommend that hypopnea be defined as a 30% or greater reduction in the airflow associated with either ≥3% decrease in oxyhemoglobin saturation, or an arousal from sleep (AHI3%A) (8). However, Centers for Medicare and Medicaid Services (CMS), along with several other payors in the United States, utilize an alternate hypopnea definition that requires at least a 4% desaturation and does not recognize arousals for defining hypopnea (AHI4%). The reimbursement for OSA therapy from these payors is reserved for the subset of patients that meets this more stringent definition of OSA. Unfortunately, this policy systematically deprives some patients, even those with clear symptoms attributable to sleep apnea such as increased sleepiness, of appropriate therapy, since they do not meet the higher diagnostic cutoff mandated by this definition.
Much of the current status quo may be related to a lack of substantial data evaluating the impact of hypopnea events associated with less severe desaturation or arousals on diverse OSA outcomes. In contrast, several large cohort studies have established a robust relationship between OSA defined using the AHI4% definition and cardiovascular outcomes (9-11). Two large community-based longitudinal studies demonstrating an association between OSA severity and all-cause mortality, that from Sleep Heart Health Study (SHHS) cohort (3) and that from Wisconsin Sleep Cohort (5), also utilized the AHI4% definition. However, no large community-based longitudinal studies have assessed the association between OSA diagnosed using the AHI3%A definition and mortality. The current study utilized data from SHHS to assess the relationship between OSA defined by the AHI3%A at baseline and all-cause mortality over an 11-year follow up period.
Methods
Participants
The Sleep Heart Health Study (SHHS) was a multicenter cohort study that investigated prospectively the relationship between OSA and cardiovascular diseases in the United States. Details of the rationale and study design have been described elsewhere (12). Recruitment began in 1995 with eventual enrollment of 6,441 participants, 40 years of age and older, from several ongoing “parent” cardiovascular and respiratory disease cohorts who were initially assembled between 1976 and 1995 (13). These “parent” cohorts consisted of the Offspring and the Omni Cohorts of the Framingham Heart Study in Massachusetts; the Hagerstown, MD, and Minneapolis, MN, sites of the Atherosclerosis Risk in Communities Study; the Hagerstown, MD, Pittsburgh, PA, and Sacramento, CA, sites of the Cardiovascular Health Study; 3 hypertension cohorts (Clinic, Worksite, and Menopause) in New York City; the Tucson Epidemiologic Study of Airways Obstructive Diseases and the Health and Environment Study; and the Strong Heart Study of American Indians in Oklahoma, Arizona, North Dakota, and South Dakota. Between 1995 and 1997, these participants underwent a home sleep evaluation that included full unattended polysomnography to determine whether they had OSA. Subsequently, they were followed for mortal events by their parent cohorts. Follow-up duration was 10.9±3.2 years (Mean±SD). As shown in Figure 1, consent was withdrawn by 134 participants from the Arizona cohort of the Strong Heart Study because of sovereignty issues after the end of the follow-up period.
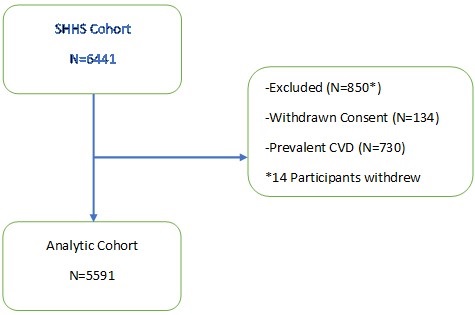
Figure 1. Diagram of Sleep Heart Health Study (SHHS) analytic cohort.
Participants with self-reported prevalent cardiovascular disease (CVD: coronary heart disease, stroke or congestive heart failure) at enrollment also were excluded. Consequently, there were 5,591 participants in the analytic cohort. Parent cohort data were used for documentation of age, height, sex and ethnicity. Co-morbid self-reported diabetes, cardiovascular disease (CVD), concurrent treatment for OSA and smoking status were ascertained from parent cohort data or from responses on health interview and sleep habit questionnaires administered on the evening of the polysomnography home visit (vide infra). Hypertension status was derived as previously described from blood pressure measurements on the night of the home visit and hypertensive medication use (14). Body mass index (BMI) was calculated as weight (kg)/height (m2).
Institutional review boards for human subjects’ research of the respective parent cohorts approved the study. Informed written consent was obtained from all participants at the time of their recruitment.
Polysomnography and Home Visit
Participants underwent overnight in-home polysomnograms using the Compumedics Portable PS-2 System (Abbottsville, Victoria, Australia) administered by trained technicians (15). The home visits were performed by two-person, mixed-sex teams in visits that lasted 1.5 to 2 hours. At the time of the home visit, blood pressure was measured manually in triplicate in a seated position after 5 minutes of rest (16). The average of the second and third measurements was used. Body weight was measured using a digital scale.
The SHHS recording montage for both the initial and follow-up sleep evaluations consisted of electroencephalogram (C4/A1 and C3/A2), right and left electrooculogram, a bipolar submental electromyogram, thoracic and abdominal excursions (inductive plethysmography bands), airflow (detected by a nasal-oral thermocouple [Protec, Woodinville, WA]), oximetry (finger pulse oximetry [Nonin, Minneapolis, MN]), electrocardiogram and heart rate (using a bipolar electrocardiogram lead), body position (using a mercury gauge sensor), and ambient light (on/off, by a light sensor secured to the recording garment). Equipment and sensors were applied and calibrated during the evening home visit by a study certified technician. In the morning, the equipment and the data stored in real time on PCMCIA cards, were retrieved and downloaded to the computers of each respective clinical site. The data were locally reviewed, and then forwarded to a central reading center (Case Western Reserve University, Cleveland, OH). Comprehensive descriptions of polysomnography scoring and quality-assurance procedures have been previously published (15,17). In brief, sleep was scored according to guidelines developed by Rechtschaffen and Kales (18). Strict protocols were maintained to ensure comparability among centers and technicians. Intra-scorer and inter-scorer reliabilities were high (17).
The apnea hypopnea index (AHI) was calculated for each participant using the AASM recommended definition of hypopnea. Thus, hypopneas were identified if the amplitude of a measure of flow or volume (detected by the thermocouple or thorax or abdominal inductance band signals) was reduced discernibly (at least 25% lower than baseline breathing) for at least 10 seconds, did not meet the criteria for apnea and the event was associated with either a ≥3% oxygen desaturation from baseline or terminated with electroencephalographic evidence of an arousal. An apnea was defined as a complete or almost complete cessation of airflow, as measured by the amplitude of the thermocouple signal, lasting at least 10 seconds.
Statistical Analyses
Mean and standard deviation were used to provide an overall description of the data used in the analyses. For analyses using the AHI, each participant’s AHI was assigned to one of 4 OSA severity categories: No OSA (AHI <5 /hour), Mild (AHI ≥5 and <15 /hour), Moderate (AHI ≥15 and < 30/hour) and Severe (AHI ≥30). For some analyses, because values for AHI were extremely left skewed, a natural log transformation was performed to express AHI as a continuous factor in the form of lnAHI+0.1. To nullify the impact of 0 values of the AHI, 0.1 was added to the ln function. Mortality rates were computed by dividing the number of deaths by accumulated person-years at risk.
Analysis of variance was used to test for differences within continuous variables and 2 was employed for categorial variables. A Kaplan-Meir plot was computed to assess the overall relationship between severity of OSA and mortality. Cox proportional hazards regression models were calculated to examine the association between AHI as a categorical and continuous factor and mortality. Covariates included in the models were sex, race, age, BMI, cholesterol, high density lipoprotein (HDL), hypertension and/or diabetes and smoking status. Consistent with a previous study assessing mortality in SHHS, age was dichotomized into those <70 and those ≥ 70 years (3). Race was stratified as non-Hispanic White or other. Smoking was recategorized into those who were current or former smokers and those who were never smokers. Prevalent hypertension or self-reported diabetes was expressed as present or absent. Three models were constructed: Model 1 adjusted for age, race and sex, Model 2 adjusted for covariates in Model 1 plus BMI and Model 3 adjusted for covariates in Models 1 and 2 plus cholesterol, HDL, hypertension/diabetes and smoking status.
Analyses were performed using IBM SPSS Statistics v27 (Armonk, NY). The survival package in R was used to obtain the Kaplan Meir plot. A p value of <0.05 was considered statistically significant.
Results
Demographic and clinical characteristics of the cohort stratified by AHI are shown in Table 1.
Table 1. Baseline Characteristics Stratified by Apnea Hypopnea Indexa,b
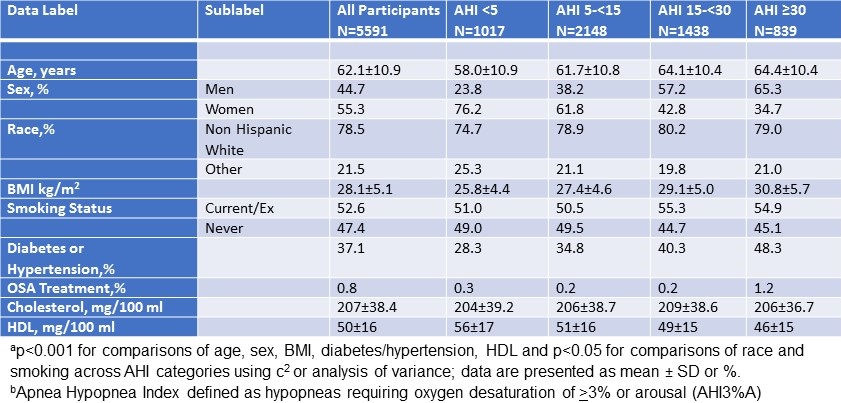
Age and BMI increased across AHI strata as well as the % of men, current/ex-smokers, diabetic/hypertensives and non-Hispanic Whites. In contrast, HDL decreased. No changes were observed for cholesterol or % receiving OSA treatment.
Figure 2 depicts the Kaplan-Meir plot of survival over ~11 years of follow-up stratified by AHI categories.
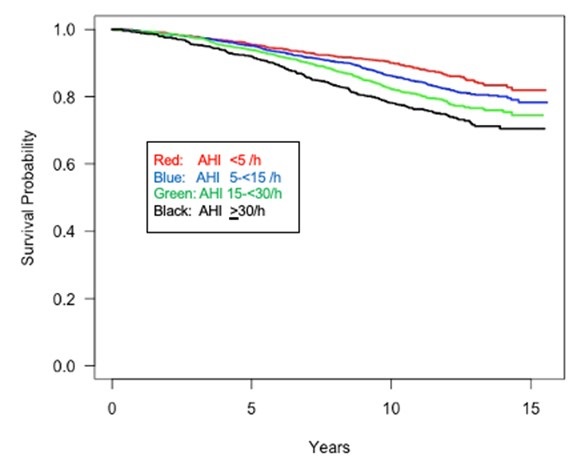
Figure 2. Kaplan Meir plot of survival stratified by apnea hypopnea (AHI) severity.
There was a clear reduction in survival with apparent differences related to AHI severity. However, because several covariates also impacted survival across AHI strata, multivariate proportional hazard modelling was employed as shown in for all participants as shown in Table 2.
Table 2. Hazard Ratios (95% confidence intervals) for All-Cause Mortality
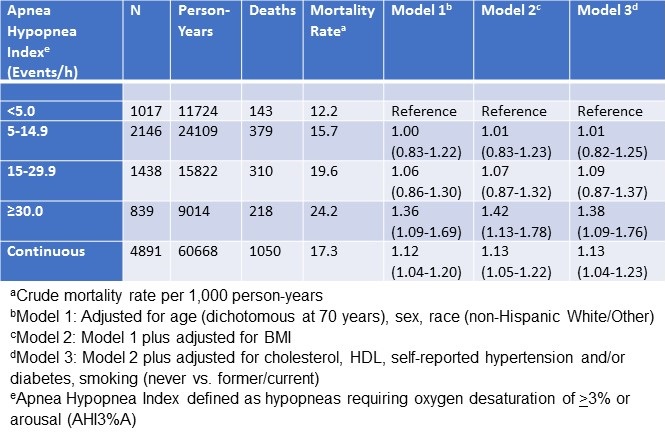
There were 1,050 deaths with full covariate data available for analysis. For the categorical modelling, there was an increase in the hazard ratio as the AHI severity increased, but this was only statistically significant at an AHI ≥30 /h (HR: 1.36, 95% CI: 1.09-1.69). Increasing model complexity did not alter this finding. A model using AHI as a continuous factor also demonstrated a significant association between severity of AHI and increasing mortality in a fully adjusted model. A sensitivity analysis where concurrent OSA treatment was included also did not change this relationship.
Because previous analyses have demonstrated differences in mortality between men and women, sex stratified analyses were performed as shown in Table 3.
Table 3. Hazard Ratios (95% confidence intervals) for All-Cause Mortality Stratified by Sex
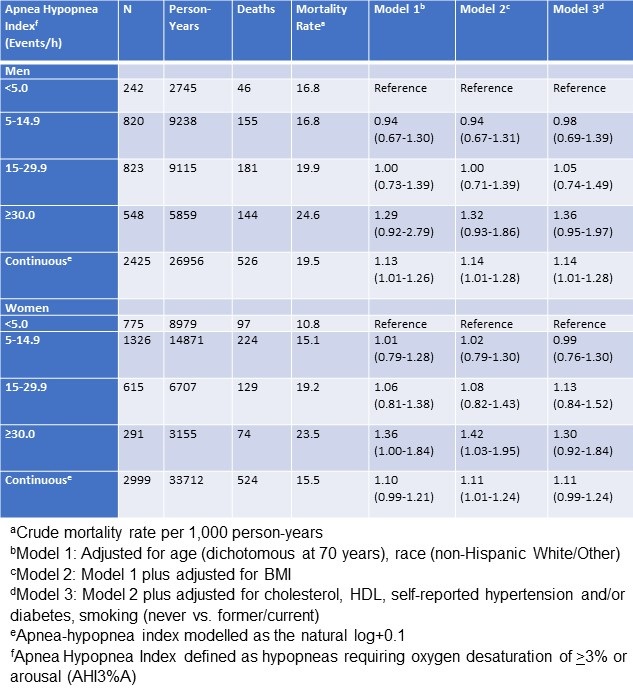
These findings confirmed that in men AHI severity in both categorical and continuous analyses was associated with increased mortality. As observed in the combined analyses, this was only statistically significant in the continuous analysis (HR: 1.14, 95% CI: 1.01-1.28) although strong trends were noted in the categorical analyses in all models. In women, however, the relationship between AHI severity and mortality was less robust. In demographic (Model 1) and demographic/anthropometric (Model 2) adjusted analyses, an AHI ≥30 /h was associated with increased mortality, but this observation was attenuated and lost statistical significance in the fully adjusted categorical and continuous models.
Table 4 shows age stratified analyses comparing those <70 years to those ≥70 years of age.
Table 4. Hazard Ratios (95% confidence intervals) for All-Cause Mortality Stratified by Age at 70 years

In those who were <70 years, AHI severity was strongly associated with increased mortality. Although this finding was statistically significant only at AHI ≥30 /h in the fully adjusted model, it was significant at AHI 15-29.9/h in less complex models (HR: 1.45, 95% CI: 1.03-2.04) and approached significance in the fully adjusted model (HR: 1.41, 95% CI: 0.98-2.00). In contrast, AHI severity was not found to be associated with increased mortality among those ≥70 years of age in either categorial or continuous models.
Of the 1,050 deaths used in the proportional hazard models, 258 (24.7%) were classified as related to CVD. In analyses restricted to CVD deaths, a Kaplan-Meir plot (not shown) indicated a reduction in survival with increasing OSA severity (Log Rank 2 = 11.2-20.4 for comparisons vs. AHI <5 /h, p<.001). However, in fully adjusted proportional hazard models, no differences in survival attributable to OSA were observed.
Discussion
The current study demonstrated using the AHI3%A definition of hypopnea, a significant association between increasing severity of AHI and all-cause mortality in a model adjusted for relevant anthropometric and demographic factors and clinical co-morbidities. In stratified analyses, this association was more robust among men than in women, and those below 70 years of age compared to the older subjects.
Notably, some earlier studies have demonstrated an increase in mortality associated with OSA. An 18-year follow-up from Wisconsin cohort revealed a significantly increased hazard ratio for all-cause mortality and cardiovascular mortality in severe OSA (5). Punjabi et al. (3) used data from SHHS and demonstrated an increase in all-cause mortality with severe OSA, particularly in men aged 40–70, during an average follow-up period of 8.2 years. Both these studies utilized the AHI4% criteria for OSA diagnosis. Similarly, Martínez-García (19) utilized AHI4% criteria in a clinic population of 939 elderly (median follow-up, 69 months) and found HR of 2.25 for cardiovascular mortality in the untreated severe OSA group. A study from Denmark included 22,135 OSA patients found that male gender, age>40 years, diabetes (types 1 and 2), hypertension, and heart failure were associated with greater mortality (criteria for hypopnea not specified (6). Marin et al. (10) also noted increased fatal and non-fatal cardiovascular events in men with untreated severe OSA diagnosed using the AHI4% criteria during a mean 10.1 years follow-up period. A meta-analysis with 11,932 patients from 6 prospective observational studies found severe OSA to be a strong independent predictor for cardiovascular and all-cause mortality (4). Finally, a meta-analysis of 27 cohort studies included 3,162,083 participants showed higher all-cause mortality in severe OSA and lower mortality in CPAP-treated than in untreated patients (7). Virtually all of these aforementioned studies utilized a definition of OSA requiring a minimum 4% oxygen desaturation to identify a hypopnea.
To our knowledge, our study is the first large community-based study to assess the association between OSA diagnosed using the AHI3%A criteria and mortality. Severe OSA was associated with a higher mortality, especially in those <70 years of age, and in men. Consistent with our findings, an earlier study in a clinical population of over 10,000 adults observed OSA diagnosed utilizing AHI3%A criteria predicted incident sudden cardiac death (20). The higher mortality risk in men and in younger people is similar to that reported in other analyses from this database using AHI4% criteria (3,21). Our results provide evidence that the more liberal AHI3%A criteria is associated with increased all-cause mortality thus providing further justification for its use in identifying persons with OSA who may benefit from treatment.
We observed that approximately 25% of the deaths in our analytic cohort were attributable to CVD. Data from the Wisconsin Sleep Cohort indicate that excess mortality associated with OSA over a 18 year follow-up is partially related to CVD (5). Our unadjusted analyses are consistent with this observation. However, our study did not have sufficient power in adjusted models to replicate it.
There are several factors that could explain the association between OSA and increased mortality. OSA increases the risk for hypertension, cardiovascular disease, diabetes, and stroke and can, thus, increase mortality. Hypoxemic burden has been suggested to be a conspicuous factor conferring an increased mortality risk (22). Other factors, however, may also play a notable role. Analyses from 5,712 participants revealed that short respiratory event duration, a marker for low arousal threshold, was associated with higher mortality risk (21). The authors hypothesized that the shorter event duration reflected greater “arousability”, resulting in greater sleep fragmentation, shorter sleep, and excess sympathetic tone, and hence increased mortality. Arousals are associated with an increase in the sympathetic activity and a decrease in the parasympathetic activity and data support their role in the development of hypertension.
From a clinical perspective, utilizing the AHI4% criteria in lieu of AHI3%A to identify persons as having OSA impacts those who are classified as having OSA by the latter standard, but not the former. Using the SHHS database, we found that 36.1% of individuals fall into this category. Importantly, similar to persons who were classified as having OSA by both criteria, we observed that this group who were designated as having OSA by only AHI3%A criteria had increased rates of prevalent and incident hypertension (23,24). There also was a significant association with CVD (25). Combined with these previous studies, the current analyses demonstrating increased mortality associated with OSA defined by AHI3%A criteria provide evidence that use of this more liberal definition will benefit patients.
This study has several strengths. SHHS is large, ethnically diverse cohort, making the results more generalizable. The cohorts were community-based, obviating any referral bias. Polysomnography, the gold standard diagnostic test for OSA, was performed on all individuals. The substantive database allowed controlling for multiple confounders. Finally, the participants were followed for an ample time with the average follow-up period of 11 years.
The study also has some limitations. First, being a community derived cohort, the severity of OSA seen in SHHS was generally mild to moderate. The outcomes, including mortality, would be expected to be worse in a clinical cohort with higher severity of sleep apnea. Secondly, while the current study included a substantial number of potential covariates in the models, residual confounding from other factors may have occurred. Thirdly, the severity of OSA may have changed over the follow up period. Fourthly, while the follow-up period of the study was long, it is possible that an even longer follow-up period may have allowed a better estimate of the long-term impact of OSA on mortality. Finally, although the study demonstrated increased mortality risk, elucidation of the mechanisms thereof was beyond the scope of this study.
In conclusion, the current study demonstrated in a large community-based cohort that even OSA defined by a more liberal AHI3%A is associated with increased mortality. Considering the adverse outcomes associated with OSA, a restrictive definition that excludes these persons from warranted OSA therapy is potentially deleterious to overall health with significant individual and healthcare implications.
References
- Javaheri S, Barbe F, Campos-Rodriguez F, et al. Sleep Apnea: Types, Mechanisms, and Clinical Cardiovascular Consequences. J Am Coll Cardiol. 2017 Feb 21;69(7):841-858. [CrossRef] [PubMed]
- Budhiraja R, Parthasarathy S, Quan SF. Endothelial dysfunction in obstructive sleep apnea. J Clin Sleep Med. 2007 Jun 15;3(4):409-15. [PubMed]
- Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O'Connor GT, Rapoport DM, Redline S, Resnick HE, Robbins JA, Shahar E, Unruh ML, Samet JM. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009 Aug;6(8):e1000132. [CrossRef] [PubMed]
- Ge X, Han F, Huang Y, Zhang Y, Yang T, Bai C, Guo X. Is obstructive sleep apnea associated with cardiovascular and all-cause mortality? PLoS One. 2013 Jul 25;8(7):e69432. [CrossRef] [PubMed]
- Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, Stubbs R, Hla KM. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008 Aug;31(8):1071-8. [PubMed]
- Jennum P, Tønnesen P, Ibsen R, Kjellberg J. Obstructive sleep apnea: effect of comorbidities and positive airway pressure on all-cause mortality. Sleep Med. 2017 Aug;36:62-66. [CrossRef] [PubMed]
- Fu Y, Xia Y, Yi H, Xu H, Guan J, Yin S. Meta-analysis of all-cause and cardiovascular mortality in obstructive sleep apnea with or without continuous positive airway pressure treatment. Sleep Breath. 2017 Mar;21(1):181-189. [CrossRef] [PubMed]
- Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012 Oct 15;8(5):597-619. [CrossRef] [PubMed]
- Gottlieb DJ, Yenokyan G, Newman AB, O'Connor GT, Punjabi NM, Quan SF, Redline S, Resnick HE, Tong EK, Diener-West M, Shahar E. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010 Jul 27;122(4):352-60. [CrossRef] [PubMed]
- Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005 Mar 19-25;365(9464):1046-53. [CrossRef] [PubMed]
- Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000 May 11;342(19):1378-84. [CrossRef] [PubMed]
- Quan SF, Howard BV, Iber C, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997 Dec;20(12):1077-85. [PubMed]
- Lind BK, Goodwin JL, Hill JG, Ali T, Redline S, Quan SF. Recruitment of healthy adults into a study of overnight sleep monitoring in the home: experience of the Sleep Heart Health Study. Sleep Breath. 2003 Mar;7(1):13-24. [CrossRef] [PubMed]
- Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D'Agostino RB, Newman AB, Lebowitz MD, Pickering TG. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000 Apr 12;283(14):1829-36. [CrossRef] [PubMed]
- Redline S, Sanders MH, Lind BK, Quan SF, Iber C, Gottlieb DJ, Bonekat WH, Rapoport DM, Smith PL, Kiley JP. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep. 1998 Nov 1;21(7):759-67. [PubMed]
- O'Connor GT, Caffo B, Newman AB, Quan SF, Rapoport DM, Redline S, Resnick HE, Samet J, Shahar E. Prospective study of sleep-disordered breathing and hypertension: the Sleep Heart Health Study. Am J Respir Crit Care Med. 2009 Jun 15;179(12):1159-64. [CrossRef] [PubMed]
- Whitney CW, Gottlieb DJ, Redline S, Norman RG, Dodge RR, Shahar E, Surovec S, Nieto FJ. Reliability of scoring respiratory disturbance indices and sleep staging. Sleep. 1998 Nov 1;21(7):749-57. [CrossRef] [PubMed]
- Rechtschaffen A. A manual for standardized terminology, techniques and scoring system for sleep stages in human subjects. Brain information service. Washington, DC : United States Government Printing Office, 1968.
- Martínez-García MA, Campos-Rodríguez F, Catalán-Serra P, Soler-Cataluña JJ, Almeida-Gonzalez C, De la Cruz Morón I, Durán-Cantolla J, Montserrat JM. Cardiovascular mortality in obstructive sleep apnea in the elderly: role of long-term continuous positive airway pressure treatment: a prospective observational study. Am J Respir Crit Care Med. 2012 Nov 1;186(9):909-16. [CrossRef] [PubMed]
- Gami AS, Olson EJ, Shen WK, Wright RS, Ballman KV, Hodge DO, Herges RM, Howard DE, Somers VK. Obstructive sleep apnea and the risk of sudden cardiac death: a longitudinal study of 10,701 adults. J Am Coll Cardiol. 2013 Aug 13;62(7):610-6. [CrossRef] [PubMed]
- Butler MP, Emch JT, Rueschman M, Sands SA, Shea SA, Wellman A, Redline S. Apnea-Hypopnea Event Duration Predicts Mortality in Men and Women in the Sleep Heart Health Study. Am J Respir Crit Care Med. 2019 Apr 1;199(7):903-912. [CrossRef] [PubMed]
- Azarbarzin A, Sands SA, Stone KL, et al. The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: the Osteoporotic Fractures in Men Study and the Sleep Heart Health Study. Eur Heart J. 2019 Apr 7;40(14):1149-1157. [CrossRef] [PubMed]
- Budhiraja R, Javaheri S, Parthasarathy S, Berry RB, Quan SF. The Association Between Obstructive Sleep Apnea Characterized by a Minimum 3 Percent Oxygen Desaturation or Arousal Hypopnea Definition and Hypertension. J Clin Sleep Med. 2019 Sep 15;15(9):1261-1270. [CrossRef] [PubMed]
- Budhiraja R, Javaheri S, Parthasarathy S, Berry RB, Quan SF. Incidence of hypertension in obstructive sleep apnea using hypopneas defined by 3 percent oxygen desaturation or arousal but not by only 4 percent oxygen desaturation. J Clin Sleep Med. 2020 Oct 15;16(10):1753-1760. [CrossRef] [PubMed]
- Quan SF, Budhiraja R, Javaheri S, Parthasarathy S, Berry RB. The association between obstructive sleep apnea defined by 3 percent oxygen desaturation or arousal definition and self-reported cardiovascular disease in the Sleep Heart Health Study. Southwest J Pulm Crit Care. 2020;21(4):86-103. [CrossRef]
Abbreviations
- AASM American Academy of Sleep Medicine
- AHI Apnea hypopnea index
- AHI3%A Apnea hypopnea index defined using a hypopnea definition requiring a minimum 3% O2 desaturation or arousal
- AHI4% Apnea hypopnea index defined using a hypopnea definition requiring a minimum 4% O2 desaturation
- BMI Body mass index
- CMS Centers for Medicare and Medicaid Services
- CVD Cardiovascular disease
- HDL High density lipoprotein
- HR Hazard ratio
- OSA Obstructive sleep apnea
- SHHS Sleep Heart Health Study
Acknowledgements
The Sleep Heart Health Study was supported by National Heart, Lung and Blood Institute cooperative agreements U01HL53940 (University of Washington), U01HL53941 (Boston University), U01HL53938 (University of Arizona), U01HL53916 (University of California, Davis), U01HL53934 (University of Minnesota), U01HL53931 (New York University), U01HL53937 and U01HL64360 (Johns Hopkins University), U01HL63463 (Case Western Reserve University), and U01HL63429 (Missouri Breaks Research). A list of SHHS investigators, staff and their participating institutions is available on the SHHS website, http://jhuccs1.us/shhs/details/investigators.htm.
Cite as: Budhiraja R, Quan SF. Long-term all-cause mortality risk in obstructive sleep apnea using hypopneas defined by a ≥3 percent oxygen desaturation or arousal. Southwest J Pulm Crit Care. 2021;23(1):23-35. doi: https://doi.org/10.13175/swjpcc025-21 PDF

Reader Comments