Medical Image of the Month: Pulmonary Aspergillus Overlap Syndrome Presenting with ABPA, Multiple Bilateral Aspergillomas
 Tuesday, March 2, 2021 at 8:00AM
Tuesday, March 2, 2021 at 8:00AM 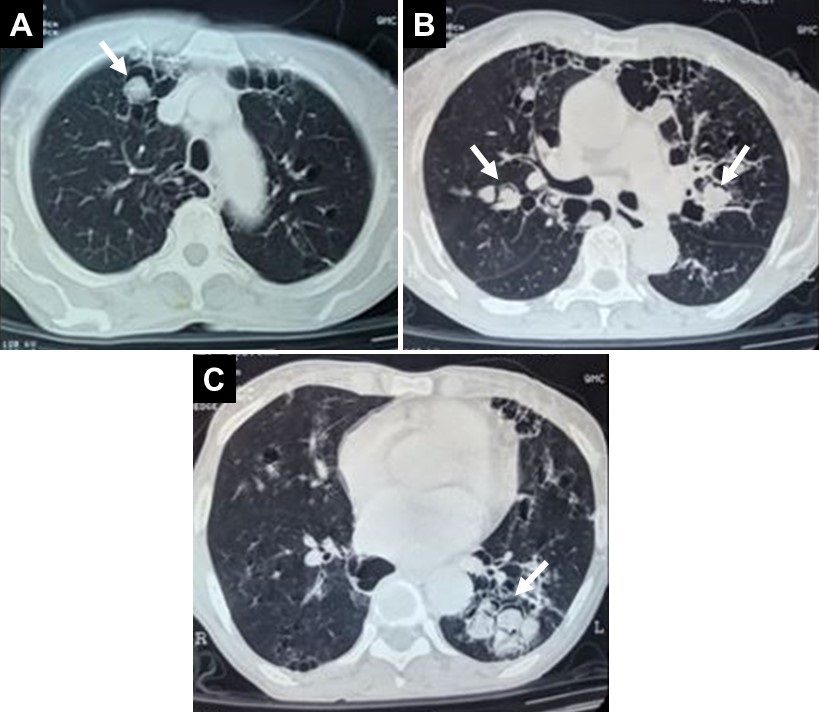
Figure 1. Representative images from thoracic CT scan in soft tissue windows showing multiple Aspergillomas (arrows).
Introduction
Aspergillus is a ubiquitous fungal organism that causes a variety of pulmonary manifestations, both in immune-competent and immune-compromised patients. It can vary from simple colonization, Aspergilloma, ABPA to Chronic Pulmonary Aspergillosis (CPA) and Invasive Pulmonary Aspergillosis (IPA) (1). ABPA is the most frequently recognized manifestation of allergic aspergillosis, caused by the immunological reactions mounted against Asp. fumigatus. Aspergillomas are rounded conglomerates of fungal hyphae, fibrin, mucus and cellular debris that arise in pulmonary cavities, as a late manifestation of CPA. Chronic pulmonary aspergillosis (CPA) is a long-term aspergillus infection of the lung. The most common form of CPA is chronic cavitary pulmonary aspergillosis (CCPA), which untreated may progress to chronic fibrosing pulmonary aspergillosis. Aspergillus overlap syndrome is defined as the occurrence of more than one form of aspergillus disease (e.g., ABPA with Aspergilloma, ABPA progressing to IPA etc.) in a single individual.
Case Report
A 58-year-old woman, resident of Bihar presented with a 4 years history of cough with expectoration, blood stained sputum on coughing, breathlessness on exertion associated with wheezing, frequent on and off episodes of fever and weight loss. She also gave history of repeated attacks of cold. She received anti-tuberculous therapy (ATT) for 9 months, prescribed on clinical and chest x-ray basis; but there was no improvement. Due to repeated attacks of haemoptysis, she was referred to our hospital for further management. She gave history of taking analgesics and steroids on and off for 20 years, for joint pains from local practitioners although Rheumatoid factor was negative. There was no other significant medical or surgical illness in the past. At the time of presentation, on clinical examination, bilateral wheeze was noted. Occasional crackles were heard on auscultation over chest bilaterally. Sputum direct smear and MGIT for Acid fast bacilli were negative. Chest X-ray showed patchy infiltrates, bronchiectatic changes, and cavities in both lungs. Sputum for AFB was negative. ELISA Test for HIV was negative. Blood examination in September 2015 showed leukocytosis with eosinophilia (TLC = 16220/mm3, DLC = N66L23M6E4.7, AEC = 770/µL). Serum Total IgE was 393.31IU/mL (0-200 IU/mL). Specific IgE for Aspergillus fumigatus was negative but Serum precipitins for Aspergillus fumigatus were positive. Sputum fungal culture at the same time grew Aspergillus fumigatus. CECT Chest showed scattered cystic bronchiectatic lesions in bilateral lungs with mycetoma formation in few of them. Peripheral air crescent formation was also present [Figure 1-3]. Peripheral pruning of bronchovascular markings was seen suggestive of emphysematous changes. Subcutaneous skin prick test was also positive for A. fumigatus and A. tamari. PFT showed mild obstruction and restriction. A diagnosis of ABPA with chronic pulmonary aspergillosis with multiple aspergillomas was made fitting into Aspergillus Overlap Syndrome (AOS). She was treated symptomatically for haemoptysis and inhaled ICS was prescribed for breathlessness. Itraconazole 200mg BD was started and ICS was continued. Follow up sputum sample for fungal culture done after 2nd and 4th month showed growth of A. fumigatus. But after 6th month, repeat sputum samples became sterile for fungal organisms indicating favorable response with azole therapy. Patient continued to have some episodes of fever and slight breathlessness and was treated symptomatically, but there was overall improvement in general condition. She gained weight and haemoptysis also abated. She was lost to follow up, but later revisited after a 10 month gap. She had continued the itraconazole. There was significant symptomatic improvement, and weight gain. Repeat blood counts showed normal TLC (8180/mm3) with DLC showing eosinophil 4.40% and AEC of 360/µL. Serum total IgE was 122 kUA/L. Repeat sputum cultures were negative for Aspergillus.
Discussion
Aspergillus is a ubiquitous fungal organism that causes a variety of pulmonary manifestations, both in immunocompetent and immunocompromised patients. It can vary from simple colonization, Aspergilloma, ABPA, to Chronic Pulmonary Aspergillosis (CPA) and Invasive Pulmonary Aspergillosis (IPA) (1).
Chronic Pulmonary Aspergillosis (CPA) was recognized as a clinical entity in 1842 (2). Several different terminologies and classifications have been proposed. Denning et al. (3) in 2003 proposed a classification dividing CPA into Chronic Necrotizing Pulmonary Aspergillosis (CNPA), Chronic Cavitary Pulmonary Aspergillosis (CCPA), and Chronic Fibrosing Pulmonary Aspergillosis (CFPA). The ERS and ESCMID now classify CPA into five entities: 1) Simple Aspergilloma, 2) CCPA, 3) CFPA, 4) Aspergillus nodule, and 5) Subacute Invasive Aspergillosis (previously CNPA) (4).
The estimated global prevalence of CPA following pulmonary TB is 1.74 million, and ranges from 7 to 20% in ABPA cases (5). In India, the annual incidence of CPA is estimated to vary from 27, 000 cases to 1,70, 000 cases (6).
The diagnostic criteria for CPA include a consistent appearance in thoracic imaging (preferably by CT), direct evidence of Aspergillus infection or an immunological response to Aspergillus spp., and exclusion of alternative diagnoses. In addition, the minimum duration of disease should be of 3 months and patients shouldn’t be immunocompromised. Immunological response usually indicates a positive Aspergillus IgG (4).
CCPA is the more common variety of CPA and is defined as one or more pulmonary cavities (with either a thin or/ thick wall) possibly containing one or more aspergillomas or irregular intraluminal material, with serological or microbiological evidence implicating Aspergillus spp., with significant pulmonary and/or systemic symptoms and overt radiological progression (new cavities, increasing peri-cavitary infiltrates or increasing fibrosis) over at least 3 months of observation (4).
The typical radiologic features of CCPA include unilateral or bilateral areas of consolidation associated with multiple expanding usually thick-walled cavities that may contain one or more aspergillomas. Cendrine et al. (7), in their study of 36 patients over 6 months found cavities in 32 (91.4%) patients which were unilateral in 21 (65.6%) and contained fungal ball in 20 (55.5%) patients.
The term Pulmonary Aspergillus Overlap syndrome is used when two or three of the Aspergillus syndromes overlap (e.g., ABPA with Aspergilloma, ABPA progressing to IPA etc.). It has been reported in few case series and reports (8). Our patient had symptoms and radiological features suggestive of CCPA, along with positive serum precipitins for Aspergillus and Aspergillus fumigatus on sputum fungal cultures. Tuberculosis was ruled out with a negative culture. The presence of cystic bronchiectatic features and positive immediate skin prick test for A. fumigatus suggest ABPA. An Aspergillus overlap syndrome can be considered due to presence of features of ABPA with multiple aspergillomas and CCPA. The presence of multifocal pulmonary aspergillomas in CPA, seen in our patient, is a rare finding in itself (9).
Various anti-fungals like itraconazole, voriconazole, posaconazole, micafungin, caspofungin and amphotericin B have all been employed in the treatment of CPA, with near similar outcomes (10). Itraconazole being cheap and easily available with fewer side effects, is commonly used. R. Aggarwal et al.. (6) in their study on Indian patients with CCPA showed that itraconazole therapy was superior to conservative management. Oral triazole therapy is now considered the standard of care (4).
In our patient, oral itraconazole therapy for 4 months rendered sputum sterile for Aspergillus. She did not require the use of oral long-term steroids. After 1 year of therapy patient showed significant clinical improvement and she remained stable for 2 years on follow-up.
Bharath Janapati DNB, Anil K Jain MD, and Priya Sharma DNB
Department of Respiratory Medicine
National Institute of Tuberculosis and Respiratory Diseases
New Delhi 110030, India
References
- Grippi MA, Elias JA, Fishman J, Kotlof RM, Pack AI. (eds). Fishman’s Pulmonary Diseases and Disorders. 5th Edition. New York, NY: McGraw Hill. 2015.
- Bennett J. On the parasitic vegetable structures found growing in living animals. Trans Royal Soc Edinburgh. 1842;15:277-9.
- Denning DW, Riniotis K, Dobrashian R, Sambatakou H. Chronic cavitary and fibrosing pulmonary and pleural aspergillosis: case series, proposed nomenclature change, and review. Clin Infect Dis. 2003 Oct 1;37 Suppl 3:S265-80. [CrossRef] [PubMed]
- Denning DW, Cadranel J, Beigelman-Aubry C, Ader F, Chakrabarti A, Blot S, Ullmann AJ, Dimopoulos G, Lange C; European Society for Clinical Microbiology and Infectious Diseases and European Respiratory Society. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J. 2016 Jan;47(1):45-68. [CrossRef] [PubMed]
- Denning DW, Pleuvry A, Cole DC. Global burden of allergic bronchopulmonary aspergillosis with asthma and its complication chronic pulmonary aspergillosis in adults. Med Mycol. 2013 May;51(4):361-70. [CrossRef] [PubMed].
- Agarwal R, Denning DW, Chakrabarti A. Estimation of the burden of chronic and allergic pulmonary aspergillosis in India. PLoS One. 2014 Dec 5;9(12):e114745. [CrossRef] [PubMed]
- Godet C, Laurent F, Bergeron A, et al. CT Imaging Assessment of Response to Treatment in Chronic Pulmonary Aspergillosis. Chest. 2016 Jul;150(1):139-47. [CrossRef] [PubMed]
- Kousha M, Tadi R, Soubani AO. Pulmonary aspergillosis: a clinical review. Eur Respir Rev. 2011 Sep 1;20(121):156-74. [CrossRef] [PubMed]
- Pendleton M, Denning DW. Multifocal pulmonary aspergillomas: case series and review. Ann N Y Acad Sci. 2012 Dec;1272:58-67. [CrossRef] [PubMed]
- Agarwal R, Vishwanath G, Aggarwal AN, Garg M, Gupta D, Chakrabarti A. Itraconazole in chronic cavitary pulmonary aspergillosis: a randomised controlled trial and systematic review of literature. Mycoses. 2013 Sep;56(5):559-70. [CrossRef] [PubMed]
Cite as: Janapati B, Jain AK, Sharma P. Medical Image of the Month: Pulmonary Aspergillus Overlap Syndrome Presenting with ABPA, Multiple Bilateral Aspergillomas. Southwest J Pulm Crit Care. 2021;22(3):76-80. doi: https://doi.org/10.13175/swjpcc002-21 PDF

Reader Comments