December 2022 Medical Image of the Month: Bronchoesophageal Fistula in the Setting of Pulmonary Actinomycosis
 Friday, December 2, 2022 at 8:00AM
Friday, December 2, 2022 at 8:00AM 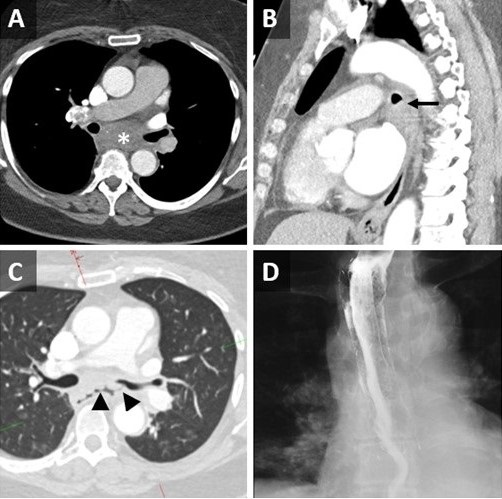
Figure 1. Axial (A) and sagittal (B) reconstructions from a contrast-enhanced chest CT demonstrates an ill-defined low-attenuation subcarinal mass (*) which causes deformity of the left mainstem bronchus (LMSB) (arrow). Axial reconstruction from a repeat contrast-enhanced CT performed 6 days later (C) demonstrates a gas-filled fistulous tract between the LMSB and esophagus through the mass (arrowheads). An esophogram (D) performed 24 hours after esophageal stent placement demonstrates occlusion of the fistula.
A 65-year-old woman, never smoker with hypothyroidism, hypertension, anxiety, and depression disorders, initially presented to the emergency department with progressive nonspecific chest discomfort for two days. She had CT Angio, which was negative for PE but showed a 4.6 cm subcarinal centrally necrotic nodal mass (Figure 1A-B). She was subsequently advised to follow up with her primary care physician. A week later, she attended our emergency department again with a new intermittent cough and one episode of non-bloody emesis. She reported a sensation of drowning with the intake of liquids and subsequent intractable coughing. Otherwise, she did not have other associated symptoms such as shortness of breath, abdominal pain, fever, sweats, or chills.
Vital signs and physical exam were unremarkable. A repeat chest CT was performed, which demonstrated internal cavitation of the subcarinal mass with fistulous communication between the lumen of the midthoracic esophagus and the proximal left mainstem bronchus posteriorly, suggestive of broncho-esophageal fistula (Figure 1C). She subsequently underwent bronchoscopy, which revealed areas of friable bronchial mucosal nodularity along the posterior membrane of the mid to distal left mainstem bronchus. Despite a thorough airway inspection, no clear fistula was observed, and no gastric or bilious material was seen within the airway. She underwent endobronchial ultrasound (EBUS) with transbronchial nodal aspiration (TBNA) of the mediastinal lymphadenopathy, which showed extensive necrotic debris and granulomatous inflammation; however, Giemsa stain was negative and no sulfur granules were observed. An upper endoscopy was performed in tandem with the bronchoscopy. The EGD identified a cratered esophageal ulcer in the mid esophagus, which was biopsied. As well, a 25 mm fistulous track was found within the ulcerated region, and thus, an esophageal stent was placed. An esophagogram performed the next day showed no evidence of a leak (Figure 1D), which is suggestive of successful occlusion of the fistula. The esophageal biopsy was negative for malignancy though it also revealed ulcerated squamous mucosa with marked acute and chronic inflammation with reactive granulation tissue.
Infectious workup included Legionella urinary antigen, Streptococcus pneumoniae urinary antigen, MRSA nasal screen, serum Aspergillus antigen, coccidiomycosis IgG/IgM (by EIA and CF/ID), QuantiFERON TB gold, and beta-D-glucan, all of which were negative. Histoplasma urinary antigen, Histoplasma and Blastomyces serum antibodies were also negative. Anaerobic cultures from lymph node aspirate later grew Actinomycetes.
Infectious disease was consulted, and the patient was started on ceftriaxone 2 g IV daily for three weeks, for pulmonary actinomyces infection, with a plan to transition to oral amoxicillin 750 mg three times a day for six months. She had a clinic follow-up appointment in eight weeks, in which she reported complete resolution of her symptoms.
Actinomycetes are branching gram-positive anaerobic bacteria and rarely cause infection, with only about 1 in 300,000 cases reported per year (1). Infections can involve any organ system, with pulmonary actinomycosis being the third most common location, representing around 15 % of the total disease cases (2). Actinomyces species are part of normal flora found in the mouth and gastrointestinal tract; therefore, it is hypothesized that pulmonary actinomycosis is caused by aspiration (3).
Diagnosis by clinical features alone can be challenging as it shares many symptoms associated with chronic infections like a low-grade fever, sputum production, cough and malaise. Therefore, it may be wrongfully diagnosed as tuberculosis, lung abscess and fungal infection. It can also often be confused with malignancy. Mabeza et al. (4) reported that around a quarter of cases with thoracic actinomyces were initially thought to have carcinoma.
Image findings of pulmonary actinomyces are also quite diverse. A retrospective study of 94 patients diagnosed with pulmonary actinomycosis pathologically over ten years in Korea revealed that the most common chest CT finding was consolidation (74.5%), mediastinal or hilar lymph node enlargement (29.8%), atelectasis (28.7%), cavitation (23.4%), ground-glass opacity (14.9%), and pleural effusion (9.6%) (5). Actinomyces can spread from the lung to the pleura, mediastinum, and chest wall. It is hypothesized that the mechanism behind their ability to travel through these anatomical barriers is due to their ability to produce proteolytic enzymes (6). Given its indolent presentation, proper diagnosis and treatment may be delayed leading to the involvement of adjacent structures and potentially life-threatening complications, including massive hemoptysis or bronchoesophageal fistula formation.
Detection of ‘sulfur’ granules histologically has been previously described as the hallmark for the diagnosis; however, they can also be found in other infections like nocardiosis (7), and they are only observed in 50% of cases; therefore, their absence does not exclude actinomycosis. Culture confirmation is typically clinically difficult because of inadequate anaerobic conditions, prior antibiotic therapy, or overgrowth of concomitant organisms (2).
The principal treatment for pulmonary actinomycosis has been penicillin; however, there are no well-established guidelines regarding the duration of antibiotic therapy. High-dose intravenous penicillin is usually used for four to six weeks, followed by six to twelve months of oral amoxicillin in most cases (9). Surgery is typically reserved for pulmonary actinomycosis complicated by abscesses, empyemas, discharging fistulas and sinuses, life-threatening hemoptysis, exclusion of malignancy, and for patients who do not respond to antibiotic therapies (10).
John Fanous MD1, Nikita Ashcherkin MD2, Michael Gotway MD3, Kenneth Sakata, MD1 and Clinton Jokerst MD3
Division of Pulmonology1, Department of Internal Medicine2, and Department of Radiology3
Mayo Clinic Arizona, Scottsdale, AZ USA
References
- Gajdács M, Urbán E, Terhes G. Microbiological and Clinical Aspects of Cervicofacial Actinomyces Infections: An Overview. Dent J (Basel). 2019 Sep 1;7(3):85. [CrossRef] [PubMed]
- Han JY, Lee KN, Lee et al. An overview of thoracic actinomycosis: CT features. Insights Imaging. 2013 Apr;4(2):245-52. [CrossRef] [PubMed]
- Park HJ, Park KH, Kim SH, Sung H, Choi SH, Kim YS, Woo JH, Lee SO. A Case of Disseminated Infection due to Actinomyces meyeri Involving Lung and Brain. Infect Chemother. 2014 Dec;46(4):269-73. [CrossRef] [PubMed]
- Mabeza GF, Macfarlane J. Pulmonary actinomycosis. Eur Respir J. 2003 Mar;21(3):545-51. [CrossRef] [PubMed]
- Kim SR, Jung LY, Oh IJ, et al. Pulmonary actinomycosis during the first decade of 21st century: cases of 94 patients. BMC Infect Dis. 2013 May 14;13:216. [CrossRef] [PubMed]
- Heo SH, Shin SS, Kim JW, Lim HS, Seon HJ, Jung SI, Jeong YY, Kang HK. Imaging of actinomycosis in various organs: a comprehensive review. Radiographics. 2014 Jan-Feb;34(1):19-33. [CrossRef] [PubMed]
- Brown JR. Human actinomycosis. A study of 181 subjects. Hum Pathol. 1973 Sep;4(3):319-30. [CrossRef] [PubMed]
- Zhang AN, Guss D, Mohanty SR. Esophageal Stricture Caused by Actinomyces in a Patient with No Apparent Predisposing Factors. Case Rep Gastrointest Med. 2019 Jan 2;2019:7182976. [CrossRef] [PubMed]
- Valour F, Sénéchal A, Dupieux C, et al. Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. 2014 Jul 5;7:183-97. [CrossRef] [PubMed]
- LoCicero J 3rd, Shaw JP, Lazzaro RS. Surgery for other pulmonary fungal infections, Actinomyces, and Nocardia. Thorac Surg Clin. 2012 Aug;22(3):363-74. [CrossRef] [PubMed]

Reader Comments