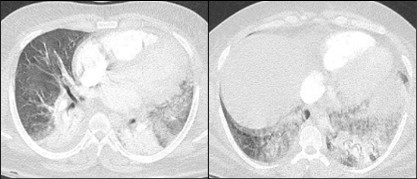
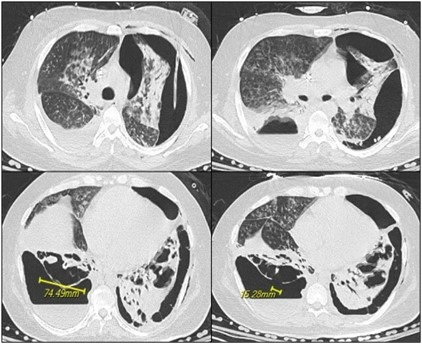
June 2023 Medical Image of the Month: Solitary Fibrous Tumor of the Pleura
 Friday, June 2, 2023 at 8:00AM
Friday, June 2, 2023 at 8:00AM 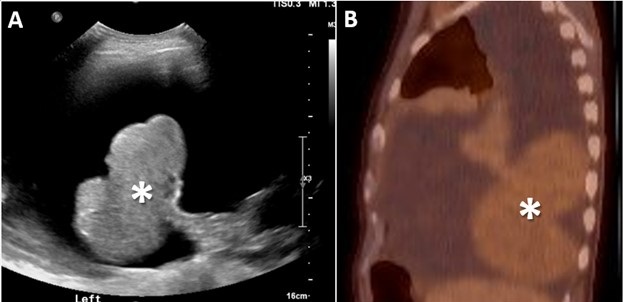 Figure 1. Posterior to anterior ultrasound image of the lower left hemithorax (A) captured during a therapeutic left-sided thoracentesis. There is a bilobed pedunculated mass (*) attached to the left lower lobe which was not noticed at the time of the procedure, but was identified in retrospect after the mass was discovered on CT. Sagittal reconstruction (B) from a 17-FDG PET-CT also demonstrate the bilobed left lower lobe mass (*). The mass demonstrates diffuse low-level FDG update suggesting relatively low metabolic activity. The appearance of the mass is very similar compared to the image capture from the thoracentesis.
Figure 1. Posterior to anterior ultrasound image of the lower left hemithorax (A) captured during a therapeutic left-sided thoracentesis. There is a bilobed pedunculated mass (*) attached to the left lower lobe which was not noticed at the time of the procedure, but was identified in retrospect after the mass was discovered on CT. Sagittal reconstruction (B) from a 17-FDG PET-CT also demonstrate the bilobed left lower lobe mass (*). The mass demonstrates diffuse low-level FDG update suggesting relatively low metabolic activity. The appearance of the mass is very similar compared to the image capture from the thoracentesis.
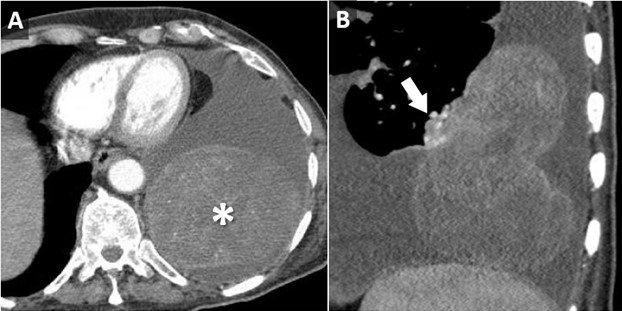 Figure 2. Axial (A) and sagittal oblique (B) reconstructions from a contrast-enhanced chest CT demonstrates a large, heterogeneously enhancing bilobed mass (*) arising from, and connected to the posterior left lower lobe via a small vascular pedicle (arrow).
Figure 2. Axial (A) and sagittal oblique (B) reconstructions from a contrast-enhanced chest CT demonstrates a large, heterogeneously enhancing bilobed mass (*) arising from, and connected to the posterior left lower lobe via a small vascular pedicle (arrow).
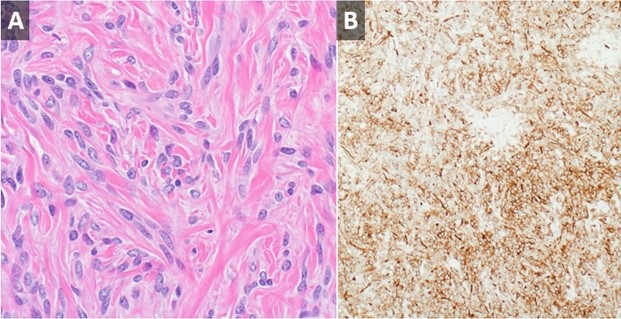 Figure 3. High-powered H & E stain (A) from surgical pathology specimen demonstrates a haphazard arrangement of spindled and ovoid cells with relatively featureless architecture. Other slides demonstrated variable myxoid stroma and areas of dilated, branching “staghorn” vessels. The cells stain strongly positive for CD34 (B) consistent with solitary fibrous tumor of the pleura.
Figure 3. High-powered H & E stain (A) from surgical pathology specimen demonstrates a haphazard arrangement of spindled and ovoid cells with relatively featureless architecture. Other slides demonstrated variable myxoid stroma and areas of dilated, branching “staghorn” vessels. The cells stain strongly positive for CD34 (B) consistent with solitary fibrous tumor of the pleura.
An 85-year-old man presented to our institution for a second opinion and for management of a recurrent left-sided pleural effusion. The patient has a history of CLL, which was diagnosed with a chest wall biopsy 4 years prior to presentation. Since that time, he has undergone chemotherapy and had a good response. In the past 18 months, the patient has had their left-sided pleural effusion drained 24 times. The patient also has a history of hypothyroidism and has had a cholecystectomy.
The patient brought multiple outside imaging studies with him for review. An image capture from a recent ultrasound-guided left thoracentesis (Figure 1A) demonstrated, in retrospect, a pedunculated left lower lobe mass. An outside PET-CT (Figure 1B) was also available, confirming the presence of this mass, which had relatively uniform, low level FDG uptake such that it evade notice on first interpretation. A CT angiogram (Figure 2) demonstrated a large, bilobed mass with heterogeneous arterial enhancement that was attached to and arising from the visceral pleura of the left lower lobe. The angiographic scanning phase demonstrated a well-developed vascular pedicle by which the mass attached to the left lower lobe. Needle biopsy (and subsequent resection) of the mass revealed a 13.5 cm solidary fibrous tumor of the pleura.
Solitary fibrous tumor of the pleura (SFTP) was first described by Klemperer and Rabin in 1931 and has undergone multiple name changes over the years, having been called benign mesothelioma, localized mesothelioma, solitary fibrous mesothelioma, pleural fibroma, submesothelial fibroma, subserosal fibroma, and localized fibrous tumor at various points in the past (1). SFTP is a rare tumor, accounting for less than 5% of tumors arising from the pleura (2). Although it can rarely arise outside the pleura (peritoneum, pericardium, meninges), it most commonly arises from the pleura. It can arise from either the visceral or parietal pleural layer and tends to have a pedunculated attachment in the case of the former with a more broad-based attachment in the case of the later (3). In the case of a SFTP arising from the visceral pleura, it’s pedunculated nature may result in a “wandering” chest mass (4).
SFTP most commonly presents incidentally, often on an imaging study. Imaging findings can be relatively nonspecific, aside from pleural origin. Probably the most salient lesson from this case is to be sure to be sure to perform a diagnostic analysis of any imaging obtained for procedural guidance. SFTP’s are probably best known for the two unusual clinical syndromes that have been described in association with them. There may be hypertrophic pulmonary osteoarthropathy (Pierre-Marie-Bamberg syndrome), which is caused by osteolysis related to the excessive release of hyaluronic acid. There may also refractory hypoglycemia (Doege-Potter syndrome), which is caused by release of insulin-like growth factor II by the tumor cells (4).
Clinton Jokerst MD, Matthew Stib MD, Carlos Rojas MD, Kristopher Cummings MD, Eric Jensen MD, Prasad Panse MD, and Michael Gotway MD
Department of Radiology, Mayo Clinic Arizona, Scottsdale, AZ USA
References
- Klemperer P, Rabin CB. Primary neoplasm of the pleura: a report of five cases. Arch Pathol. 1931;11:385-412.
- Shields TW. Localized fibrous tumors of the pleura. In: Shields TW, ed. General Thoracic Surgery. 4th ed. Baltimore, Md: Williams & Wilkins; 1994
- Robinson LA. Solitary fibrous tumor of the pleura. Cancer Control. 2006 Oct;13(4):264-9. [CrossRef] [PubMed]
- Bhardwaj H, Lindley S, Bhardwaj B, Carlile PV, Huard DR. Catch me if you can: a wandering solitary fibrous tumor of the pleura. Am J Respir Crit Care Med. 2014 Aug 1;190(3):e7-9. [CrossRef] [PubMed]
- Luciano C, Francesco A, Giovanni V, Federica S, Cesare F. CT signs, patterns and differential diagnosis of solitary fibrous tumors of the pleura. J Thorac Dis. 2010 Mar;2(1):21-5. [PubMed]