The Effect of Low Dose Dexamethasone on the Reduction of Hypoxaemia and Fat Embolism Syndrome After Long Bone Fractures
 Tuesday, January 24, 2023 at 8:00AM
Tuesday, January 24, 2023 at 8:00AM Dr. Akash K
Dr. Madhuchandra R
Department Of Orthopaedics, Karnataka Institute Of Medical Sciences, Hubli, India
Abstract
Background: A dangerous and sometimes fatal consequence of post-traumatic long bone fractures is fat embolism syndrome (FES). The reported incidence of FES ranges from 2% to 22%. FES can also lead to critical illness with fatality rates between 10 to 36%. This study's objective was to determine whether prophylaxis of the fat emboli syndrome could be achieved with lower doses of dexamethasone than had previously been recommended. Thus, prevention of respiratory insufficiency and disruption of homeostasis are essential.
Methods: A total of 583 adult cases of long bone shaft fracture patients between January 2020 to December 2021 were randomly divided into a trial group (n= 252) and a control group (n=331) by simple randomization. The trial group received dexamethasone 8mg/day for 3 days and the control group was given placebo. FES was diagnosed using Gurd’s diagnostic criteria and the FES morbidity and death rates in each group were examined.
Results: Five patients (0.151%) in the control group and 1 patient (0.39%) in the trial group developed FES but the difference was not significant (p>0.05). SpO2 values were significantly elevated in the dexamethasone-treated group compared to the control group 24 hours after admission (p<0.05) and the elevation persisted on the third post admission day (p<0.05).
Conclusion: Dexamethasone in low doses reduces post-traumatic hypoxia in patients with long bone fracture. However, our study was underpowered to show a reduction in FES.
Introduction
Fat emboli occur in all long bone fractures with the most severe resulting in fat embolism syndrome (FES). The reported incidence of FES ranges from 2% to 22% with fatality rates of 10-36% (1-3) with FES resulting in the adult respiratory distress syndrome a 50–90% mortality rate (1-3). Unfortunately, this is particularly common in young people in their second and third decades of life who sustain polytrauma and/or femur fractures in high-velocity traffic accidents (2,3). The majority of trauma patients may experience a subclinical form of FES, which manifests as hypoxaemia alone (3-6).
FES resulting in systemic symptoms is a rare clinical outcome. Following a traumatic incident, fat droplets are released into the bloodstream resulting in fat embolization. This results in immediate tissue damage as well as a systemic inflammatory response that produces symptoms in the lungs, skin, nervous system, and retina (7,8). Most instances of FES occur after trauma but rare cases of FES have been reported to occur after bone marrow transplantation, osteomyelitis, pancreatitis, alcoholic fatty liver, and even liposuction (9,10). Although the classic triad of pulmonary distress, mental status changes, and petechial rash is usually not seen, hypoxia 24 to 48 hours after pelvic or long-bone fractures is common (11-13).
FES has no pathognomonic characteristics and laboratory and radiographic findings are nonspecific (14,15). Early detection of FES may allow supportive pulmonary treatment and other life-saving interventions to stop the pathophysiologic cascade and stop clinical deterioration. The majority of curative methods created expressly for FES have failed (16,17). There have been several attempts to avoid FES since it is such a serious issue in trauma patients (4). With varying degrees of success, heparin, dextran, albumin, hypertonic glucose, aspirin, and early fracture stabilization, have all been attempted (4). Steroids have also been studied as a preventative as well as a therapeutic agent in fat embolism in various studies.
When fat droplets act as emboli and are trapped in the pulmonary microvasculature and other microvascular beds, such as the brain, they may cause clinical symptoms to appear 24-72 hours after trauma (and particularly after fractures). Embolization starts out very slowly and reaches its peak in 48 hours or more. A long-acting corticosteroid having a half-life of 36 to 72 hours is dexamethasone. This study's objective was to determine whether prophylaxis of the fat emboli syndrome could be achieved with lower doses of dexamethasone than had previously been recommended (17).
Patients and Methods
From January 2020 to December 2021, 583 adult patients between the ages of 18 and 60 with long bone fractures without a history of chronic heart, lung, liver, or renal failure were recruited from patients at KIMS Hospital Hubli. There were 211 cases observed in women and 372 cases in men. The injuries resulted from motor accidents (426), falls (127), and crush injuries (30). Fracture sites included 128 femur fractures, 285 tibia and fibula fractures, 79 humerus fractures, and 91 pelvic injuries. The patients were randomized into two groups, one receiving dexamethasone and the other receiving a placebo (Table 1).
Table 1. Demographic data
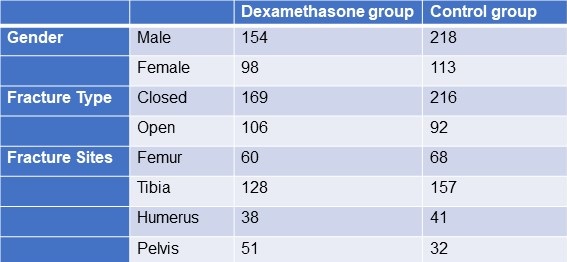
Click here to display Table 1 in a separate, enlarged window.
The following patient information was recorded: gender, age, weight, time from injury to admission, primary fracture location, type of fracture, FES morbidity, and number of fatalities. All patients received traditional medical care, early hypovolemic shock correction, fracture stabilization, and symptomatic therapy (2). The trial group received dexamethasone 8mg/day for 3 days and the control group was given placebo. All patients were monitored (heart rate, BP, SpO2 ,respiratory rate, urine output, and arterial blood gases) every 6 hours for 3 days. We considered hypoxaemia with any pO2 <70mm Hg and classified all patients in 3 categories; severe (pO2<60mm Hg), mild hypoxaemia (pO2 >60- <70 mm Hg) and normal (pO2>70mm Hg). All patients signed an informed consent form. The study was approved by the Ethics Committee of our institute hospital.
Treatment and diagnosis for FES
Patients were identified using “Gurd’s Diagnostic criteria score”(Table 2), and those whose score was 2 major or 1 major and 4 minor were diagnosis as FES.
Table 2. Gurd’s Diagnostic Criteria Score*
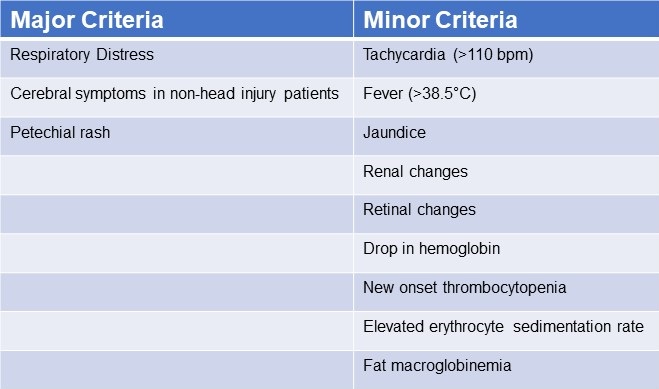
*Two major criteria or 1 major criterion and 4 minor criteria suggest a diagnosis of FES. Click here to view Table 2 in a separate and enlarged window.
Data analysis
Utilizing statistical tools, the analysis was conducted (SPSS 20.0). P< 0.05 was regarded as statistically significant when comparing the patients' age, main fracture location, fracture type, and incidence of FES using the chi-squared test and single-factor analysis of variance, respectively.
Results
FES occurred in the dexamethasone group and control group, with 1 and 5 cases, respectively (Table 3). Statistical analysis revealed that there was no statistically significant difference between the groups for sex, age, weight, injury to admission time, main fracture site, fracture type, or medication time.
Table 3. Incidence of FES
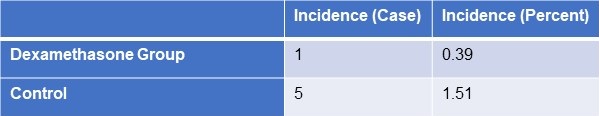 Click here to view Table 3 in a separate, enlarged window.
Click here to view Table 3 in a separate, enlarged window.
Twenty-four hours after admission, steroid treated patients displayed a statistically significant higher PaO2 value compared to the control group (p<0.05) and this difference persisted through the 3rd post admission day (p<0.05, table 4).
Table 4. Partial pressures of oxygen (in mm Hg) in patients treated with IV dexamethasone and controls.
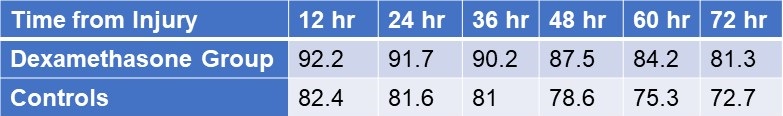
Click here to view Table 4 in a separate and enlarged window.
Discussion
Much higher dosages of dexamethasone have been used to treat some pathological conditions in order to reduce inflammation, inhibit the immune system, impact the hemopoietic system, and alter metabolism (18-28). The mechanical-chemical hypothesis of fat embolism hypothesizes that neutral triglycerides are hydrolyzed into glycerol and free fatty acids by lipoprotein lipase from the lungs. The free fatty acids lead to inflammation and endothelial damage. Corticosteroids likely act on FES by reducing this inflammation. Due to a lack of clear diagnostic markers, treating FES may prove challenging. There have been few publications on the use of adrenal steroids to prevent high-risk FES patients, although the results have been ambiguous at low doses (31). Observational clinical research revealed that short-range and high doses may be helpful in reducing plasma free fatty acid concentrations, maintaining PaO2 levels, and reducing the occurrence of long bone fractures in individuals with FES. Dexamethasone may be a more effective drug treatment for FES (32). The dose of dexamethasone used in our study was relatively small and short, and complications related to hormones such as stress ulcer, aseptic necrosis of the femoral head, and bleeding tendency did not occur. It should be noted that drug prevention must be based on early, accurate fracture fixation, early corrective hypovolemic shock, and other standard procedures (33). This is true even if drug usage in this population clearly has a preventative impact. Ashbaugh and Petty (34) suggested corticosteroid therapy for treating FES in 1966 and gave laboratory data proving its therapeutic impact in the experimental animal given an intravenously administered FFA injection. Rokkanen et al. (35) found that 5 mg/kg of dexamethasone administered at 1 and 48 h after burn injury failed to enhance nuclear translocation of the GR, and to suppress the overproduction of proinflammatory cytokines such as TNF-α and IL-1β, neither did it increase the release of anti-inflammatory cytokine IL-10. In experiments with animals, Kreis et al. (36) showed that corticosteroids increased oxygenation and lowered the pathological alterations seen in lung biopsies. Alho et al. (37) conducted research on the use of intravenous methyl prednisolone sodium succinate in the prevention of fat embolism syndrome. A total of 60 individuals with at least two fractures were included in his study (pelvic, femoral or tibial fractures).methyl prednisolone reduces signs of hypoxaemia, bilateral "snow storm" infiltrations of the lungs, petechial rash, mental disturbances, pyrexia, anemia and thrombocytopenia. Varying degrees of the syndrome were observed in two patients given methylprednisolone and in 15 patients in the control group. Babalis et al. (39) results support the prophylactic administration of methylprednisolone in small dosage to prevent post traumatic hypoxaemia and probably FES in patients with isolated lower limb long bone fractures, especially when early fracture stabilization is not possible. Therefore, every study has demonstrated the effectiveness of steroids as a preventative treatment for the fat embolism syndrome.
Although our results showed a trend towards reduction in FES after long bone fractures, the results were not statistically significant. This is likely because our study turned out to be underpowered. We had anticipated an incidence of FES between 2-20% reported in the literature rather than the 1.1% found in our study.
Conclusion
The study's objective was to determine whether prophylaxis of the fat emboli syndrome could be achieved with lower doses of dexamethasone than had previously been recommended. Among the several prophylactic drugs that have been researched so far for the fat embolism syndrome, dexamethasone have shown to be relatively beneficial. The frequency of hypoxaemia and fat emboli syndrome decreased with intravenous dexamethasone at 8 mg per day for three days. Dexamethasone is a long-acting symptoms that emerge 24-72 hours after trauma (and particularly after fractures). Fat embolization begins slowly and reaches its maximum around 48 hours.
The limitation of our study is that it lacked sufficient power to demonstrate a reduction in FES. Furthermore, no method has been developed to pinpoint precisely who could benefit from steroid prophylaxis. We based our study assuming an incidence of FES of about 5%. However, we found an incidence of only about 1.5%. The lower incidence is probably due to our use of Gurd’s criteria which is more restrictive than the criteria used in other studies. Based on our observed incidence of FES of 1.5% with a reduction to 0.4% we estimate that over 2500 patients would be needed to show a statistically significant reduction in FES.
Our study shows that hypoxaemia is reduced by a relatively low dose of dexamethasone administered for a relatively short length of time. It may prevent FES but our study was underpowered to show a difference.
Declaration
Human subjects: Consent was obtained or waived by all participants in this study. Karnataka Institute Of Medical Sciences ethics committee. issued approval 327/2020-21. The study was approved by the institutional ethics committee. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissues. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all
authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work
References
- Sproule BJ. Brady JL. Gilbert J. Studies on the Syndrome of Fat Embolization. Can Med Assoc J. 1964 May 30;90(22):1243-7. [PubMed]
- Wertzberger JL, Peltier LF. Fat embolism: the importance of arterial hypoxia. Surgery. 1968 Apr;63(4):626-9. [PubMed]
- Stürm JA, Lewis FR Jr, Trentz O, Oestern HJ, Hempelman G, Tscherne H. Cardiopulmonary parameters and prognosis after severe multiple trauma. J Trauma. 1979 May;19(5):305-18. [CrossRef] [PubMed]
- Hutchins PM, Macnicol MF. Pulmonary insufficiency after long bone fractures. Absence of circulating fat or significant immunodepression. J Bone Joint Surg Br. 1985 Nov;67(5):835-9. [CrossRef] [PubMed]
- Levy D. The fat embolism syndrome. A review. Clin Orthop Relat Res. 1990 Dec;(261):281-6. [PubMed]
- Gossling HR, Pellegrini VD Jr. Fat embolism syndrome: a review of the pathophysiology and physiological basis of treatment. Clin Orthop Relat Res. 1982 May;(165):68-82. [PubMed]
- Kwiatt ME, Seamon MJ. Fat embolism syndrome. Int J Crit Illn Inj Sci. 2013 Jan;3(1):64-8. [CrossRef] [PubMed]
- Parisi DM, Koval K, Egol K. Fat embolism syndrome. Am J Orthop (Belle Mead NJ). 2002 Sep;31(9):507-12. [PubMed]
- Scuderi CS. The present status of fat embolism. Bibliographic review. Int Surg Digest 1934; 18: 195-215.
- Gurd AR. Fat embolism: an aid to diagnosis. J Bone Joint Surg Br. 1970 Nov;52(4):732-7. [PubMed]
- Nixon JR, Brock-Utne JG. Free fatty acid and arterial oxygen changes following major injury: a correlation between hypoxaemia and increased free fatty acid levels. J Trauma. 1978 Jan;18(1):23-6. [CrossRef] [PubMed]
- Parker FB Jr, Wax SD, Kusajima K, Webb WR. Hemodynamic and pathological findings in experimental fat embolism. Arch Surg. 1974 Jan;108(1):70-4. [CrossRef] [PubMed]
- Nijsten MW, Hamer JP, ten Duis HJ, Posma JL. Fat embolism and patent foramen ovale. Lancet. 1989 Jun 3;1(8649):1271. [CrossRef] [PubMed]
- Vedrinne JM, Guillaume C, Gagnieu MC, Gratadour P, Fleuret C, Motin J. Bronchoalveolar lavage in trauma patients for diagnosis of fat embolism syndrome. Chest. 1992 Nov;102(5):1323-7. [CrossRef] [PubMed]
- White T, Petrisor BA, Bhandari M. Prevention of fat embolism syndrome. Injury. 2006 Oct;37 Suppl 4:S59-67. [CrossRef] [PubMed]
- Laterre PF, Wittebole X, Dhainaut JF. Anticoagulant therapy in acute lung injury. Crit Care Med. 2003 Apr;31(4 Suppl):S329-36. [CrossRef] [PubMed]
- Bederman SS, Bhandari M, McKee MD, Schemitsch EH. Do corticosteroids reduce the risk of fat embolism syndrome in patients with long-bone fractures? A meta-analysis. Can J Surg. 2009 Oct;52(5):386-93. [PubMed]
- McEvoy GK, Snow EK, Kester L, eds. AHFS 2002 Drug Information. Bethesda, MD: American Society of Health‐System Pharmacists; 2002.
- Chamberlain D. Emergency medical treatment of anaphylactic reactions. Project Team of the Resuscitation Council (UK). J Accid Emerg Med. 1999 Jul;16(4):243-7. [CrossRef] [PubMed]
- Niermeyer S, Kattwinkel J, Van Reempts P, et al. International Guidelines for Neonatal Resuscitation: An excerpt from the Guidelines 2000 for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care: International Consensus on Science. Contributors and Reviewers for the Neonatal Resuscitation Guidelines. Pediatrics. 2000 Sep;106(3):E29. [CrossRef] [PubMed]
- Brun-Buisson C, Brochard L. Corticosteroid therapy in acute respiratory distress syndrome: better late than never? JAMA. 1998 Jul 8;280(2):182-3. [CrossRef] [PubMed]
- Hudson LD. New therapies for ARDS. Chest. 1995 Aug;108(2 Suppl):79S-91S. [CrossRef] [PubMed]
- Meduri GU, Headley AS, Golden E, Carson SJ, Umberger RA, Kelso T, Tolley EA. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1998 Jul 8;280(2):159-65. [CrossRef] [PubMed]
- Johnson MJ, Lucas GL. Fat embolism syndrome. Orthopedics. 1996 Jan;19(1):41-8; discussion 48-9. [CrossRef] [PubMed]
- Kallenbach J, Lewis M, Zaltzman M, Feldman C, Orford A, Zwi S. 'Low-dose' corticosteroid prophylaxis against fat embolism. J Trauma. 1987 Oct;27(10):1173-6. [PubMed]
- Niewoehner DE, Erbland ML, Deupree RH, Collins D, Gross NJ, Light RW, Anderson P, Morgan NA. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study Group. N Engl J Med. 1999 Jun 24;340(25):1941-7. [CrossRef] [PubMed]
- Richards RR. Fat embolism syndrome. Can J Surg. 1997 Oct;40(5):334-9. [PubMed]
- Kubota T, Ebina T, Tonosaki M, Ishihara H, Matsuki A. Rapid improvement of respiratory symptoms associated with fat embolism by high-dose methylpredonisolone: a case report. J Anesth. 2003;17(3):186-9. [CrossRef] [PubMed]
- Han YY, Sun WZ. An evidence-based review on the use of corticosteroids in peri-operative and critical care. Acta Anaesthesiol Sin. 2002 Jun;40(2):71-9. [PubMed]
- Habashi NM, Andrews PL, Scalea TM. Therapeutic aspects of fat embolism syndrome. Injury. 2006 Oct;37 Suppl 4:S68-73. [CrossRef] [PubMed]
- Babalis GA, Yiannakopoulos CK, Karliaftis K, Antonogiannakis E. Prevention of posttraumatic hypoxaemia in isolated lower limb long bone fractures with a minimal prophylactic dose of corticosteroids. Injury. 2004 Mar;35(3):309-17. [CrossRef] [PubMed]
- Yamamoto T, Irisa T, Sugioka Y, Sueishi K. Effects of pulse methylprednisolone on bone and marrow tissues: corticosteroid-induced osteonecrosis in rabbits. Arthritis Rheum. 1997 Nov;40(11):2055-64. [CrossRef] [PubMed]
- Talbot M, Schemitsch EH. Fat embolism syndrome: history, definition, epidemiology. Injury. 2006 Oct;37 Suppl 4:S3-7. [CrossRef] [PubMed]
- Ashbaugh DG, Petty TL. The use of corticosteroids in the treatment of respiratory failure associated with massive fat embolism. Surg Gynecol Obstet. 1966 Sep;123(3):493-500. [PubMed]
- Rokkanen P, Alho A, Avikainen V, Karaharju E, Kataja J, Lahdensuu M, Lepistö P, Tervo T. The efficacy of corticosteroids in severe trauma. Surg Gynecol Obstet. 1974 Jan;138(1):69-73. [PubMed]
- Kreis WR, Lindenauer SM, Dent TL. Corticosteroids in experimental fat embolization. J Surg Res. 1973 Mar;14(3):238-46. [CrossRef] [PubMed]
- Alho A, Saikku K, Eerola P, Koskinen M, Hämäläinen M. Corticosteroids in patients with a high risk of fat embolism syndrome. Surg Gynecol Obstet. 1978 Sep;147(3):358-62. [PubMed]
- Stoltenberg JJ, Gustilo RB. The use of methylprednisolone and hypertonic glucose in the prophylaxis of fat embolism syndrome. Clin Orthop Relat Res. 1979 Sep;(143):211-21. [PubMed]
- Babalis GA, Yiannakopoulos CK, Karliaftis K, Antonogiannakis E. Prevention of posttraumatic hypoxaemia in isolated lower limb long bone fractures with a minimal prophylactic dose of corticosteroids. Injury. 2004 Mar;35(3):309-17. [CrossRef] [PubMed]

Reader Comments