October 2022 Medical Image of the Month: Infected Dasatinib Induced Chylothorax-The First Reported Case
 Sunday, October 2, 2022 at 8:00AM
Sunday, October 2, 2022 at 8:00AM 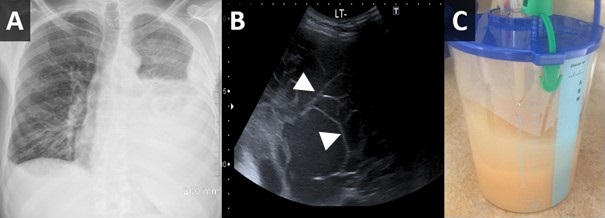
Figure 1. Upright PA chest radiograph (A) demonstrates a large left-sided pleural effusion with some lateral fluid suggesting loculation. Bedside ultrasound to guide thoracentesis (B) demonstrates multiple loculations within the effusion (arrowheads). Thoracentesis yielded 2 liters of milky white fluid (C).
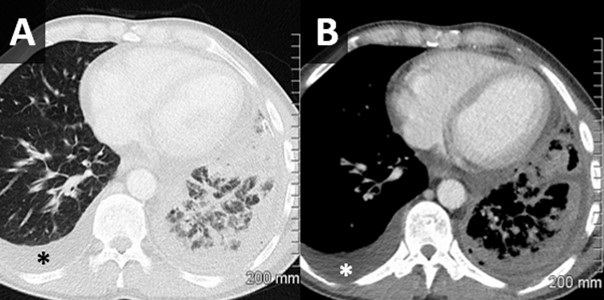
Figure 2. Axial lung window (A) and soft tissue window (B) reconstructions from a chest CT with intravenous contrast performed following thoracentesis demonstrates a circumferential irregular left-sided pleural effusion with air space disease within the left lower lobe concerning for infection. A simple-appearing right-sided effusion is noted as well (*).
Case Report
A 45-year-old man with chronic myeloid leukaemia (CML) on dasatinib presented to the emergency department with a 2-week history of dry cough, worsening shortness of breath and left-sided chest pain that had worsened on the day of presentation. On examination, oxygen saturation was 98% on 2 L nasal cannula, respiratory rate 22 bpm, pulse 77 bpm, blood pressure 117/90 mmHg and his temperature was 37.9° C (100.2 F). Examination of the left chest showed no air entry and stony dull percussion note.
Laboratory results were significant for leucocytosis with a neutrophil count of 11.2, elevated CRP of 414, mildly elevated lactate of 1.1. Initial chest X-ray showed large left-sided pleural effusion and a small volume right effusion (Figure 1A). The patient was started on IV piperacillin /tazobactam, blood cultures were obtained and the dasatinib was held.
Ultrasound-guided left thoracentesis and drain placement was performed, on ultrasound the effusion demonstrated several loculations (Figure 1B). An 18Fr drain was inserted and 2L of white purulent/milky material fluid was drained (Figure 1C). Pleural fluid analysis showed abundant neutrophils, macrophages, lymphocytes and a few reactive mesothelial cells. Cytological analysis was negative for malignant cells. The fluid was exudative by Light’s criteria as total protein was 52.9 g/l and serum protein was 77 g/l with the ratio 0.68. Triglyceride level was 2.0 mmol/l and fluid cholesterol was 1.6 mmol/L indicative of chylothorax.
Over time, pleural cultures were positive for beta haemolytic Strep group C/G sensitive to penicillin G and erythromycin and both fungal and tuberculosis cultures were negative. Blood cultures were negative. Antimicrobial therapy was deescalated to Penicillin G. A subsequent chest CT (following intra-pleural fibrinolytic therapy) showed small left basal effusion with overlying consolidation and no occlusive lesion identified (Figure 2). After 9 days the pleural drain was removed, and the patient had no reaccumulation of their chylothorax. The patient remained clinically well and was discharged after a course of four weeks of antibiotics. At a 2 week follow up the patient was asymptomatic and had a normal physical exam. His inflammatory markers were back to normal CRP was 0.5 and WBC count was 6.5.
Discussion
Chylothorax is accumulation of chyle into the pleural space related to obstruction or disruption of the thoracic duct. It is a rare condition that may arise from diverse etiologies broadly categorized as traumatic or non-traumatic/spontaneous (1). Chylothorax is widely believed to be inherently bacteriostatic, with rare incidence of infected chylous effusions affecting a wide variety of patients with different causative organisms and a mostly benign course (2).
Dasatinib is a second-generation tyrosine kinase inhibitor that is recommended as the first-line therapy for newly diagnosed chronic myeloid leukaemia (CML) or acute lymphoblastic leukaemia (ALL) with positive Philadelphia chromosome (Ph+) or as an alternative for the failure of prior therapy for CML. Dasatinib is known to cause fluid retention which commonly presents as an exudative pleural effusion (3), chylothorax is rarely seen with 7 cases in total related to dasatinib use were published in the literature (4).
This is the first reported case of infected chylothorax among the population using dasatinib. Infected chylothorax in general is rare, affecting wide variety of patients with different organisms and mostly benign course (2). In this report the patient was stable on presentation and showed good response to antibiotics, chest drainage, holding of dasatinib and dietary fat restriction. Given the loculated appearance of the fluid the patient benefited from a dose of thrombolysis, which was reported as an option in such a scenario (5).
In patients with CML on dasatinib presenting with pleural effusion, the medication should be considered as one of the possible causes. Furthermore, infected chylothorax should be considered in the deferential diagnosis as a source of sepsis in patients presenting with a sepsis-like clinical picture and pleural effusion. The case also reflects the importance of bedside ultrasound in both clinically examining the patients and as a guide for thoracentesis and guidance for therapy.
Mortada Mohammed1 MD MRCPI, Mohanad Abdelrahim2 MD, Keshav Sharma3 MD MRCPI
1Respiratory medicine registrar Wexford General Hospital, Wexford, Ireland
2Medical Senior House officer Wexford General Hospital, Wexford, Ireland
3Consultant Respiratory and General Medicine Physician, Wexford General Hospital, Wexford, Ireland
References
- McGrath EE, Blades Z, Anderson PB. Chylothorax: aetiology, diagnosis and therapeutic options. Respir Med. 2010 Jan;104(1):1-8. [CrossRef] [PubMed]
- Eubank L, Gabe L, Kraft M, Billheimer D. Infected chylothorax: a case report and review. Southwest J Pulm Crit Care. 2018 Aug 25;17(2):76–84. [CrossRef]
- Keating GM. Dasatinib: A Review in Chronic Myeloid Leukaemia and Ph+ Acute Lymphoblastic Leukaemia. Drugs. 2017 Jan;77(1):85-96. [CrossRef] [PubMed]
- Chen B, Wu Z, Wang Q, Li W, Cheng D. Dasatinib-induced chylothorax: report of a case and review of the literature. Invest New Drugs. 2020 Oct;38(5):1627-1632. [CrossRef] [PubMed]
- Nair SK, Petko M, Hayward MP. Aetiology and management of chylothorax in adults. Eur J Cardiothorac Surg. 2007 Aug;32(2):362-9. [CrossRef] [PubMed]