July 2016 Imaging Case of the Month
 Sunday, July 3, 2016 at 8:00AM
Sunday, July 3, 2016 at 8:00AM Michael B. Gotway, MD
Department of Radiology
Mayo Clinic Arizona
Scottsdale, AZ USA
Imaging Case of the Month CME Information
Members of the Arizona, New Mexico, Colorado and California Thoracic Societies and the Mayo Clinic are able to receive 0.25 AMA PRA Category 1 Credits™. Completion of an evaluation form is required to receive credit and a link is provided on the last panel of the activity.
0.25 AMA PRA Category 1 Credit(s)™
Estimated time to complete this activity: 0.25 hours
Lead Author(s): Michael B. Gotway, MD. All Faculty, CME Planning Committee Members, and the CME Office Reviewers have disclosed that they do not have any relevant financial relationships with commercial interests that would constitute a conflict of interest concerning this CME activity.
Learning Objectives:
As a result of this activity I will be better able to:
- Correctly interpret and identify clinical practices supported by the highest quality available evidence.
- Will be better able to establsh the optimal evaluation leading to a correct diagnosis for patients with pulmonary, critical care and sleep disorders.
- Will improve the translation of the most current clinical information into the delivery of high quality care for patients.
- Will integrate new treatment options in discussing available treatment alternatives for patients with pulmonary, critical care and sleep related disorders.
Learning Format: Case-based, interactive online course, including mandatory assessment questions (number of questions varies by case). Please also read the Technical Requirements.
CME Sponsor: University of Arizona College of Medicine at the Arizona Health Sciences Center.
Current Approval Period: January 1, 2015-December 31, 2016
Financial Support Received: None.
Clinical History: An 18-year-old non-smoking man with a previous diagnosis of Ehlers-Danlos syndrome presented with mild shortness of breath and new cough. Physical examination was normal. The patient was afebrile.
Laboratory data were remarkable except for a mildly elevated white blood cell count of 11 x 109 cells/L. Serum chemistries were within normal limits. Oxygen saturation on room air was 97%.
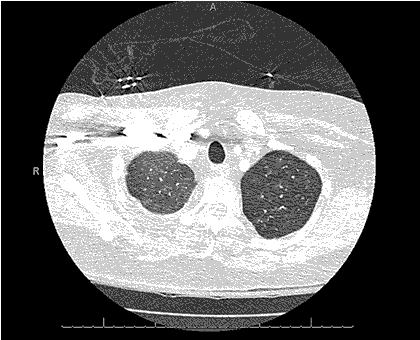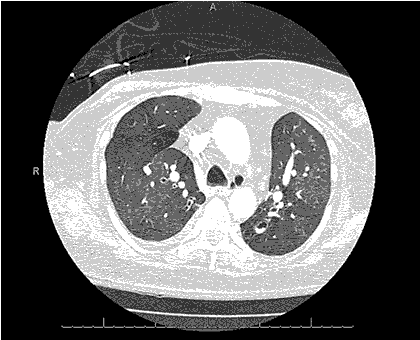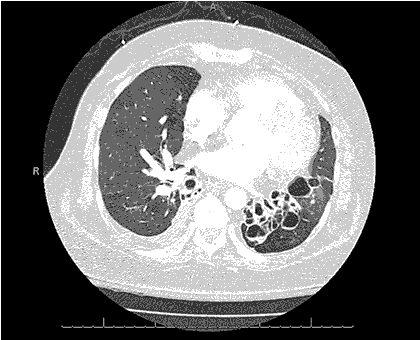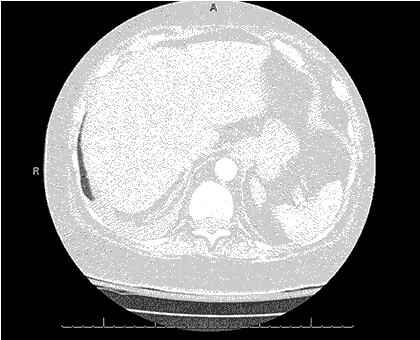
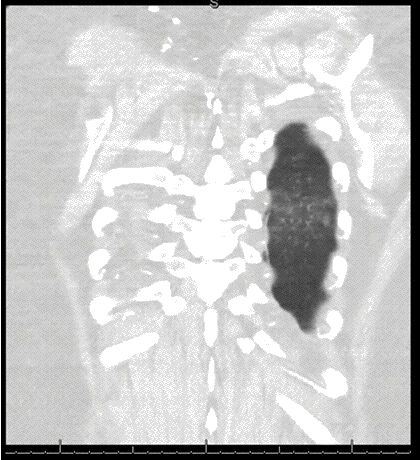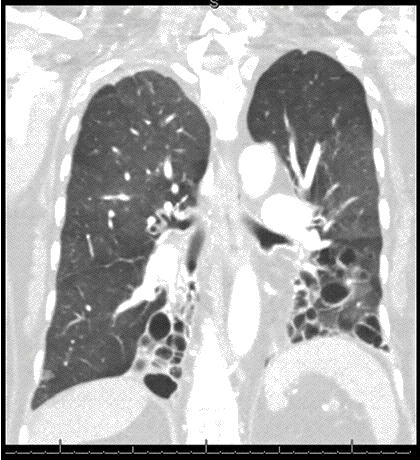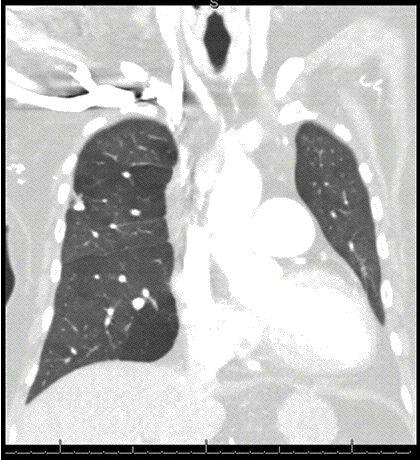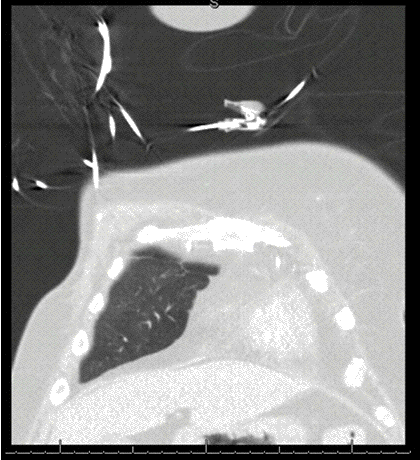
Frontal chest radiography (Figure 1) was performed.
Figure 1. Frontal chest radiography
A previous comparison chest radiograph from 3 years earlier (Figure 2) is shown as well.

Figure 2. Frontal and lateral chest radiography from 3 years earlier.
Which of the following statements regarding the chest radiograph is most accurate? (Click on the correct answer to proceed to the second of seven panels)
Cite as: Gotway MB. July 2016 imaging case of the month. Southwest J Pulm Crit Care. 2016:13(1):15-26. doi: http://dx.doi.org/10.13175/swjpcc061-16 PDF