Cough and Pleural Disease in a Burmese Immigrant – A Masquerader
 Thursday, June 14, 2012 at 11:31AM
Thursday, June 14, 2012 at 11:31AM George M. Solomon, MD1
Eric Schmidt, MD1,2
Randall Reves, MD3
Carolyn Welsh, MD1
Department of Medicine, Divisions of Pulmonary Sciences and Critical Care Medicine, University of Colorado Denver1
Denver Health Medical Center2
Denver Metro Tuberculosis Control Program, Denver Public Health Department3
Corresponding Author: George M. Solomon, MD
Research Building 2
9th Floor12700 E. 19th Ave
Aurora, Colorado 80045
Phone 303-398-1392
Financial Disclosures: No authors report any financial conflicts to disclose
Abstract
We present a case of a 58 year old Burmese male who presented to our center with progressive pulmonary and constitutional symptoms after treatment for pulmonary tuberculosis. Our investigation revealed peripheral and bronchogenic eosinophilia and clinical features consistent with progressive pulmonary paragonimiasis. After serological confirmation of the diagnosis, the patient had resolution of symptoms with praziaquantel therapy for the condition. This case highlights the importance of considering this diagnosis when there is a possibility of undercooked shellfish exposure especially in immigrants from endemic areas for paragonimiasis where raw shellfish is more commonly ingested.
Case Presentation
A 58 year old Burmese male was referred for evaluation of cough and pleural abnormalities on a chest radiograph. The patient had arrived in Boston as a Burmese refugee the previous year. Upon arrival in the United States, he was found to have a right middle lobe infiltrate, multiple cavitary nodules on chest CT, three negative sputum AFB smears and 10 mm induration on Mantoux tuberculin skin testing. He was treated with rifampin, isoniazid, pyrazinamide, and ethambutol for 2 months with resolution of the cavitary nodules but developed an increasing right pleural opacity. Sputum cultures were negative for mycobacteria.
He moved to Denver, Colorado where an additional four months of isoniazid and rifampin were completed for presumed culture negative pulmonary tuberculosis. At the end of treatment he noted an increase in his persistent cough and fatigue associated with low grade fevers. A repeat chest radiograph now revealed a small right-sided pleural effusion (Figure 1).

Figure 1. Chest radiograph demonstrating right-sided small pleural effusion
Assessment at the pulmonary clinic revealed persistent cough and a 6.8 kg weight loss. Physical examination was pertinent for a temperature of 99.0 ºF, dullness to percussion and crackles in the right lung. The patient reported occasional tobacco use and reported occasional raw seafood consumption in Burma. He denied other medical history at this time except for untreated hypertension.
Laboratory investigation was pertinent for a white blood cell count of 10,000 per µl with a differential of 22% eosinophils and normal platelet and hemoglobin levels. Anti-nuclear antibodies (ANA), anti-neutrophil cytoplasmic antibodies (ANCA), and rheumatoid factor levels as well as a comprehensive metabolic profile and coagulation labs were all normal.
Computed tomography of the chest at the time of presentation to the pulmonary clinic compared to the one a year earlier in Boston are shown in Figure 2.
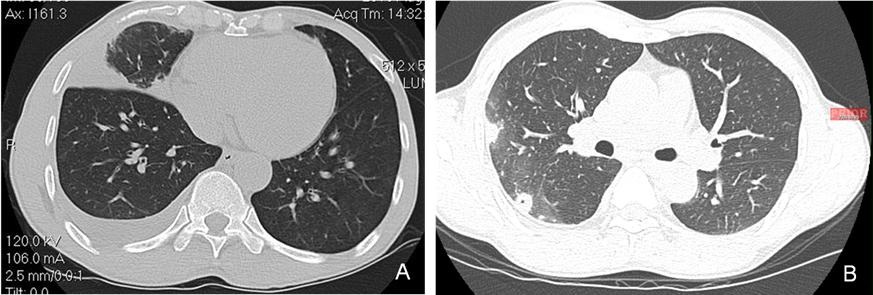
Figure 2. A. Computed Tomography image of the chest demonstrating right middle lobe opacification and pleural effusion at presentation to the pulmonary clinic. B. Computed Tomography image of the chest at time of initial treatment in Boston demonstrating right-sided cavitary disease and pleural process.
Fiberoptic bronchoscopy with bronchoalveolar lavage (BAL) revealed 960 nucleated cells, 68% of which were eosinophils. Routine cultures grew normal oral flora and were negative for actinomyces, fungi and mycobacteria. Wet prep for oocysts and larvae was negative.
Ultrasound revealed only a small loculated pleural effusion, precluding thoracentesis.
Because of suspicion for parasitic illness, a filarial antibody panel was also sent and was negative. Paragonimus antibody immunoblot assay was positive.
Case Follow-up
The patient was treated with praziquantel 25 mg/kg orally three times daily for 3 days with improvement in symptoms and radiographic abnormalities as show in Figure 3.
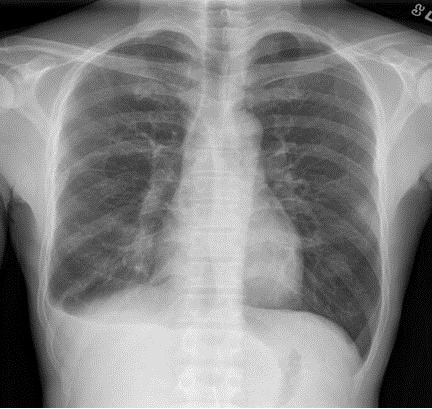
Figure 3. Chest radiograph following treatment with praziquantel demonstrating resolution of resolving right-sided pleural effusion and right-sided infiltrates.
Discussion
Paragonimiasis results from infection by one of the more than fifty species of the genus paragonimus (most commonly P. westermanii). There are approximately 2.5 million cases annually reported in endemic areas, mostly in Indo-China and sub-Saharan Africa. In the western world, most cases are reported in immigrants from endemic regions where undercooked shellfish are culturally consumed. Approximately 50-70% of infections are initially diagnosed as tuberculosis in the U.S. (1), as demonstrated in this case.
The lifecycle of the organism begins with early ingestion and early disease characterized by cough, fever, and pleuritic chest pain resulting from a transdiaphragmatic spread of larvae into the pleural space from the abdomen. Prominent features of this “early” disease are pneumothorax or pleural effusion and peripheral eosinophilia (2). Additionally, transient pulmonary infiltrates may be observed. This presentation may help to explain the response of parenchymal abnormalities in response to the anti-tuberculosis treatments. In fact, the “response” to treatment may have been a consequence of the natural history of early stage paragonimiasis.
The failure of resolution of pleural disease in this case to anti-tuberculosis drugs should have alerted clinicians to consider alternative diagnoses. The progressive pleural disease in this case while on anti-tuberculosis drugs highlights progression of paragonimiasis pulmonary disease. “Late” stage lung disease results from mature fluke inhabitation in the lung parenchyma. During this phase, patients typically resolve their peripheral eosinophilia and fever but may have persistent dark brown hemoptysis. Radiographic features in this stage are varied and include parenchymal mass-like lesions or chronic pleural lesions (2).
If paragonimiasis is suspected, diagnosis is most readily made by serological evaluation. Heretofore, microscopic evaluation of oocysts and larvae from sputum or BAL yielded a diagnosis in only 50-75% of cases (3). Serological evaluations including immunoblot assays for P. Westermanii antibodies have a reported sensitivity of 96% and specificity of 99% (4) and ELISA-based assays have a reported sensitivity of 92% and specificity of 90% (5); thereby, these assays have therefore largely supplanted other microscopic evaluation given the superior diagnostic performance
Treatment regimens are nearly 100% effective in cure for pulmonary disease. Typical regimens include three days of praziquantel therapy at 25mg/kg given three times daily. It is additionally important to counsel patients and their families on avoidance of raw seafood as well as contact prophylaxis, as cross-contamination from soiled utensils can result in illness to others (6). A recent case series of locally-acquired paragonimiasis from undercooked river shellfish in the U.S. acquired from undercooked shellfish in restaurants (7) further highlights the importance of considering this diagnosis especially if the history of potential exposure is substantiated, thus raising awareness of paragonimiasis infection as a potential public health hazard in food/beverage establishments.
In summary, paragonimiasis is a relatively common infection in endemic areas of the world. Infection is often mistaken as tuberculosis in immigrants to the western world. However, knowledge of the clinicopathologic features of the disease should lead to appropriate consideration and treatment for at-risk patients.
References
- Kagawa FT. Pulmonary paragonimiasis. Seminars in Respiratory Infections 1997;12:149-158.
- Mukae H, Taniguchi H, Matsumoto N, Iiboshi H, Ashitani J, Matsukura S, Nawa Y. Clinicoradiologic features of pleuropulmonary paragonimus westermani on Kyusyu Island, Japan. Chest 2001;120:514-520.
- Khan R, Sharma OP. Bronchial lavage in tropical pneumonias. Curr Opin Pulm Med 2007;13:225-229.
- Slemenda SB, Maddison SE, Jong EC, Moore DD. Diagnosis of paragonimiasis by immunoblot. Am J Trop Med Hyg 1988;39:469-471.
- Imai J. Evaluation of elisa for the diagnosis of paragonimiasis westermani. Trans R Soc Trop Med Hyg 1987;81:3-6.
- Johnson RJ, Jong EC, Dunning SB, Carberry WL, Minshew BH. Paragonimiasis: Diagnosis and the use of praziquantel in treatment. Reviews of infectious diseases 1985;7:200-206.
- Human paragonimiasis after eating raw or undercooked crayfish --- Missouri, July 2006-September 2010. MMWR 2010;59:1573-1576.
Reference as: Solomon GM, Schmidt E, Reves R, Welsh C. Cough and pleural disease in a Burmese immigrant-a masquerader. Southwest J Pulm Crit Care 2012;4:205-10. (Click here for a PDF version of the manuscript)

Reader Comments