Social Media: A Novel Engagement Tool for Miners in Rural New Mexico
 Friday, April 6, 2018 at 8:00AM
Friday, April 6, 2018 at 8:00AM Shreya Wigh1
William Cotton Jarrell, CMSP3
Elizabeth Kocher, MPH1
Roger Karr2
Xin Wang, MS1
Akshay Sood, MD, MPH1,2
1University of New Mexico Health Sciences Center School of Medicine
Albuquerque, NM, USA
2Miners Colfax Medical Center
Raton, NM, USA
3Peabody New Mexico Services
Grants, NM, USA
Abstract
Background: New Mexico miners usually live in rural areas. As compared to urban areas, rural areas in the United States demonstrate a lower use of the Internet and lower adoption of new technologies such as the smartphone and social media. Our study objective was to examine the use of these technologies among miners in rural New Mexico. Our long-term goal is to utilize these technologies to increase our program’s engagement with miners to provide medical screening and education services. Methods: We anonymously surveyed 212 miners at two town hall meetings in rural New Mexico communities, predominantly Hispanic and American Indian, in 2017. We then compiled that data in a Research Electronic Data Capture (REDCap) database and performed a statistical analysis using Statistical Analysis Software (SAS). IRB approval was obtained. Results: 60.8% of the 212 surveyed miners reported using social media. Among social media users, 88.4% reported using Facebook. Most miners expressed willingness to use social media to keep in contact with other miners (51.2% overall) or to receive information about our miners’ program services (53.9% overall); and social media users were more likely to do so than non-users (p<0.001 for both analyses). Additionally, 79.7% of miners who owned a smartphone utilized it for texting. Conclusions: A majority of miners in rural New Mexico report use of social media and express willingness to use social media to network with other miners and with our program. The adoption of these communication technologies by rural New Mexico miners in our study is comparable or superior to that reported by rural Americans overall. It is possible to utilize this newer technology to increase program engagement with miners.
Introduction
New Mexico miners usually live in rural and medically underserved areas and suffer from multiple chronic diseases, particularly dust related lung diseases or pneumoconiosis. Rural counties in northern New Mexico have among the highest mortality rates for silicosis and pneumoconiosis, including coal workers’ pneumoconiosis, in the United States (1). To address this challenge, Miners’ Colfax Medical Center and the University of New Mexico have partnered in a federally funded medical screening program for rural miners. As compared to urban areas, those who live in rural areas reportedly have a lower use of the Internet and are less willing to adopt new communication technologies such as the smartphone and social media (2). We have previously published that the primary source of information about miners’ health related activities for attendees at our miners’ health screening programs are traditional routes of communication such as a relative, friend, and community newspaper or flyer (3). Traditional media is, however, a one-way communication system that doesn’t create program engagement or work towards promoting word-of-mouth - the hallmark of social media (4). Our programs could utilize social media to promote awareness, encourage miner engagement, and increase the spread of accurate health messaging among New Mexico miners. Serving older, less educated, poorer, racial/ethnic minority, miners living in geographically remote and medically underserved rural areas of New Mexico may however affect the use and effectiveness of this communication tool.
The objective of our study was to examine the use of Internet-based smartphone and social media technology among miners in rural New Mexico. We hypothesized a low usage rate of these novel communication technologies among rural miners in New Mexico. Our long-term goal is to use these technologies to increase bidirectional engagement with miners with our federally funded Black Lung and Radiation Exposure Screening and Education Programs that currently provide medical screening, health care, and education services to coal and uranium miners in New Mexico.
Methods
Study design: This is a cross sectional survey of 212 miners, mostly coal miners, at two town hall meetings held in rural and medically underserved communities of Grants and Socorro, New Mexico, in 2017. These communities are predominantly American Indian and Hispanic respectively. The town hall meetings were held in conjunction with mobile health screening clinics for miners.
Survey creation: We created a survey on the use of the smartphone and social media, which asked construct-specific questions with either Yes/No responses or multiple choices. Examples of questions included whether miners would be willing to use social media to stay in touch with the mining community and if they had access to a computer with internet. The questions were formatted for an eighth-grade vocabulary, since our previous studies have shown that 57.2% of New Mexico miners do not complete high school education (3).
Survey administration: The paper copy of the survey was given to miners to fill out during the town hall meeting by the mine safety officer, on a voluntary and anonymous basis.
Analytic and database strategy: We compiled the survey data into a Research Electronic Data Capture (REDCap) database. We compared characteristics between social media users with social media non-users. Statistical analysis included an analysis of frequency distributions and Chi-square test, using Statistical Analysis Software (SAS 13.0, Cary, NC). A p-value less than 0.05 was considered statistically significant. We obtained human Institutional Review Board (IRB) approval for research exempt status (HRPO 14-058). The study was sponsored by Health Resource Services and Administration (HRSA) and Patient Centered Outcomes Research Institute (PCORI).
Results
60.8% of the 212 miners surveyed reported using social media. Among the social media users, 88.4% reported using Facebook, 27.9% reported using Instagram, and 26.4% reported using Snapchat. Social media users reported utilizing the technology for an average of 47.9 ± 134.3 (SD) minutes daily, for approximately 6.0 ± 4.4 (SD) years. Most miners expressed willingness to use social media to keep in contact with other miners (51.2% overall) or to receive information about our miners’ program services (53.9% overall); and social media users were more likely to do so than non-users (p<0.001 for both analyses, Table 1).
Table 1. Difference in characteristics between self-reported social media users and nonusers, among rural miners in New Mexico.
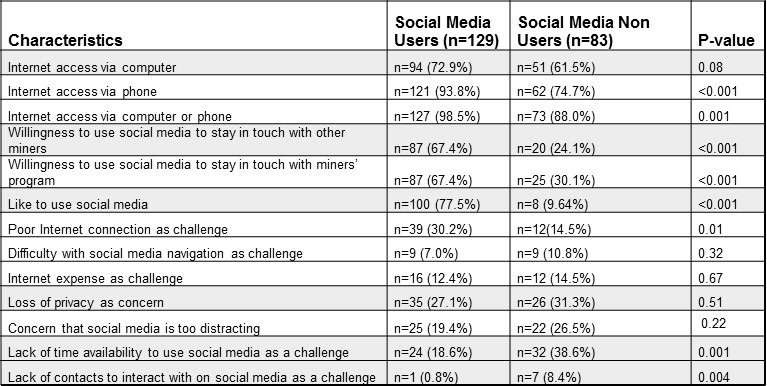
86.3% of the miners surveyed also reported possessing a smart phone (93.8% versus 74.7% of the social media users and non-users respectively; p<0.001). 79.7% of miners owning a smartphone utilized it for texting (91.5% versus 61.5% of social media users versus nonusers respectively; p<0.001).
94.3% of rural miners reported having access to the Internet. Social media users were more likely to report having Internet access via computer or via phone than non-users (p = 0.08 and <0.001 respectively, Table 1). 24.0% of all miners however reported poor Internet connection as a challenge, and as compared to nonusers, social media users were more likely to report this challenge (p=0.01). 13.2% of all miners complained of the high expense of the Internet and the social media user status did not predict this characteristic (p=0.67). There was also no difference between the two groups with respect to the reported difficulty in navigating social media sites (p=0.32).
Discussion
Based on our results, we conclude that the majority of miners in rural New Mexico use Internet-based smartphone and social media technologies and are willing to use social media to network with other miners or programs that deliver health services to miners. We found that Facebook was the most popular social media site. The adoption of these communication technologies by rural New Mexico miners in our study is comparable or superior to that reported by rural Americans overall. This suggests that it is possible to use smartphone texting and social media technology to increase bidirectional program engagement with miners in rural New Mexico.
In 2017, the proportion of US population with a social media profile was variably estimated at 69-81% (5-7). Rural Americans in the US were approximately 8% less likely to use social media than urban Americans (2). The market leader in social media was Facebook, used by 68% and 79% of all and online American adults respectively (7). In our study, 60.8% of the rural miners reported using social media and 53.8% reported using Facebook, which is comparable to that reported in other US rural communities. In 2017, the proportion of American adults who owned a smartphone was 83%, 78%, and 65% for urban, suburban, and rural locations respectively (8). In comparison, 86.3% of rural miners in our study reported possessing a smartphone, indicating a higher level of smartphone possession than that reported by rural Americans overall. In 2017-2018, 89% of all American adults used the Internet (9). In an earlier survey from November 2016, 81% of rural Americans used the Internet, as compared to 89% of urban Americans (10). 63% of rural Americans had a broadband Internet connection at home, 10 percentage points less likely than Americans overall (10). In comparison, 94.3% of rural New Mexico miners in our study reported having access to the Internet, indicating a higher level of Internet access than that reported by rural Americans overall. Contrary to our initial hypothesis, we found that rural New Mexico miners in our study reported adoption of newer communication technologies at a level that was comparable or superior to that reported by rural Americans overall.
Racial/ethnic and health status-related disparities exist with respect to Internet access in the U.S. (9). However, among those with Internet access, these characteristics do not affect their social media use (11). New Internet-based technologies including smartphone and social media, may be changing the communication pattern throughout the U.S. and the world but this change has not been well studied, particularly in rural areas (11). Potential overarching benefits of social media for health communication are (1) increased interactions with others, (2) more available, shared, and tailored information, (3) increased accessibility and widening access to health information, (4) peer/social/emotional support, (5) public health surveillance, and (6) potential to influence health policy (12). Our findings indicate that social media can similarly be used for health communication purposes among rural miners in New Mexico. Our HRSA-funded miners’ health and benefits programs in New Mexico have established a social media platform to provide rural miners with information on our clinical programs, research, education and other interventions as well as to provide opportunities for bidirectional engagement between the program and miners as well as among miners themselves. Our program has also launched a social media literacy campaign for miners, with the help of a rural mine safety officer.
Currently there is a limited amount of literature evaluating the use of social media for sustained engagement of diverse communities in health promotion (13,14). For instance, the Youth Voices Research Group has reported creating novel opportunities to engage young people to explore health topics ranging from tobacco use, food security, mental health, and navigation of health services, by combining social organizing with arts-informed methods for creative expression, using information technology (14). Creating opportunities for engagement alone is however insufficient. The information exchanged needs to be monitored for quality and reliability, users’ confidentiality and privacy need to be maintained (12), and its impact evaluated. Use of social media in health promotion in underserved populations, such as indigenous populations in Australia, is associated with limited evidence of benefit (15). Online social network health behavior interventions are reported to have small effect sizes, often statistically nonsignificant, with high participant attrition and low fidelity (16). It is therefore necessary for our program to critically evaluate the role and effectiveness of these new technologies in health promotion and health care for our population of rural miners.
The strength of our study includes inclusion of miners from rural and predominantly Hispanic and American Indian communities. Limitations of our study include small sample size and lack of information on individual demographic characteristics. Although our study was limited to New Mexico, our findings may be generalizable to other rural and medically underserved areas of the United States outside of New Mexico.
Conclusions
Most miners in rural New Mexico have Internet access, use smartphones and social media, and are willing to use social media to network with other miners or programs that deliver health services to miners. Rural New Mexico miners in our study report adoption of newer communication technologies at a level that is comparable or superior to that reported by rural Americans overall. This study provides preliminary information on a potential and novel way in which rural mining communities and miners’ health and benefits programs can engage with each other to promote miners’ health by assisting in clinical programs, research, education and other interventions. Miners’ program may consider interactive blogging, photograph elicitation, and video documentaries, alongside real-world social media projects, to promote this engagement. Potential barriers in rural miners include low social media literacy and poor Internet connection. Low social media literacy can however be addressed by targeted education of miners. Emerging areas of research include evaluating the effectiveness of the use of smartphones and social networking platforms such as Facebook, in building effective interventions for health promotion and providing healthcare for miners in rural communities.
Acknowledgments
SW, WCJ, EK, RK, KW, AS made substantial contributions to the conception or design of the work; SW, WCJ, EK, RK, KW, AS made substantial contributions to the acquisition, analysis, or interpretation of data for the work. SW, WCJ, EK, RK, KW, AS made substantial contribution towards drafting the work or revising it critically for important intellectual content. SW, WCJ, EK, RK, KW, AS provided the final approval of the version to be published. SW, WCJ, EK, RK, KW, AS agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Dwyer-Lindgren L, Bertozzi-Villa A, Stubbs RW, et al. Trends and Patterns of Differences in Chronic Respiratory Disease Mortality Among US Counties, 1980-2014. JAMA. 2017;318(12):1136-49. [CrossRef] [PubMed]
- Perrin A. Social Networking Usage: 2005-2015. Pew Research Center. 2015. Available at: http://www.pewinternet.org/2015/10/08/2015/Social-Networking-Usage-2005-2015/, last accessed on March 28, 2018.
- Evans K, Lerch S, Boyce TW, et al. An innovative approach to enhancing access to medical screening for miners using a mobile clinic with telemedicine capability. J Health Care Poor Underserved. 2016;27(4A):62-72. [CrossRef] [PubMed]
- Hausman A. Social media versus traditional media. 2014. Available at https://www.hausmanmarketingletter.com/social-media-versus-traditional-media/, accessed on January 9, 2018.
- Statistica – The Statistics Portal. Percentage of U.S. population with a social media profile from 2008 to 2017. 2018. Available at https://www.statista.com/statistics/273476/percentage-of-us-population-with-a-social-network-profile/, accessed on March 28, 2018.
- Perrin A. Social Media Fact Sheet. Pew Research Center. 2018. Available at: http://www.pewinternet.org/fact-sheet/social-media/, last accessed on March 28, 2018.
- Perrin A. Social Media Update 2016. Pew Research Center. 2016. Available at: http://www.pewinternet.org/2016/11/11/social-media-update-2016/, last accessed on March 28, 2018. 2016.
- Statistica – The Statistics Portal. Share of adults in the United States who owned a smartphone from 2011 to 2017, by location. 2018. Available at https://www.statista.com/statistics/195003/percentage-of-us-smartphone-owners-by-geographic-location/; accessed on March 28, 2018. 2018.
- Perrin A. Internet/Broadband Fact Sheet. Pew Research Center. 2018. Available at: http://www.pewinternet.org/fact-sheet/internet-broadband/, last accessed on March 28, 2018.
- Perrin A. Digital gap between rural and non-rural America persists. 2017. Pew Research Center. Available at: http://www.pewresearch.org/fact-tank/2017/05/19/digital-gap-between-rural-and-nonrural-america-persists/, last accessed on March 28, 2018.
- Chou WY, Hunt YM, Beckjord EB, Moser RP, Hesse BW. Social media use in the United States: implications for health communication. J Med Internet Res. 2009;11(4):e48. [CrossRef] [PubMed]
- Moorhead SA, Hazlett DE, Harrison L, Carroll JK, Irwin A, Hoving C. A new dimension of health care: systematic review of the uses, benefits, and limitations of social media for health communication. J Med Internet Res. 2013;15(4):e85. [CrossRef] [PubMed]
- Yonker LM, Zan S, Scirica CV, Jethwani K, Kinane TB. "Friending" teens: systematic review of social media in adolescent and young adult health care. J Med Internet Res. 2015;17(1):e4. [CrossRef] [PubMed]
- Norman CD, Yip AL. eHealth promotion and social innovation with youth: using social and visual media to engage diverse communities. Studies in health technology and informatics. 2012;172:54-70. [PubMed]
- Brusse C, Gardner K, McAullay D, Dowden M. Social media and mobile apps for health promotion in Australian Indigenous populations: scoping review. J Med Internet Res. 2014;16(12):e280. [CrossRef] [PubMed]
- Maher CA, Lewis LK, Ferrar K, Marshall S, De Bourdeaudhuij I, Vandelanotte C. Are health behavior change interventions that use online social networks effective? A systematic review. J Med Internet Res. 2014;16(2):e40. [CrossRef] [PubMed]
Cite as: Wigh S, Jarrell WC, Kocher E, Karr R, Wang X, Sood A. Social media: A novel engagement tool for miners in rural New Mexico. Southwest J Pulm Crit Care. 2018;16(4):206-11. doi: https://doi.org/10.13175/swjpcc017-18 PDF