Vanessa Yap, MD1
Diahann Wilcox, APRN, DNP1
Richard ZuWallack, MD2
Debapriya Datta, MD1
1Division of Pulmonary & Critical Care Medicine
University of CT Health Center
Farmington, CT USA
2Division of Pulmonary & Critical Care Medicine
St Francis Hospital & Medical Center
Hartford, CT USA
Abstract
Introduction: Chronic obstructive pulmonary disease (COPD) results in 700,000 hospitalizations annually in the United States and 12-25% of patients are readmitted within 30 days of hospital discharge. A simple scoring system to risk-stratify these patients would be useful in allocating scarce resources.
Objective: The objectives of this study were to identify possible predictor variables to develop a clinically-useful instrument that can predict 30-day hospital readmissions in COPD patients.
Methods: Fifty patients hospitalized for a COPD exacerbation at two hospitals over a one-month period were studied prospectively. Demographics, disease severity, symptoms, functional status, psychological, and co-morbidity variables were assessed during the hospitalization. Patients were contacted telephonically thirty days post-discharge to determine readmission. Baseline variables were tested as predictors of 30-day readmissions.
Results: Mean age was 71 ± 11 years; 77% were female, 60% had Medical Research Council dyspnea 3 or 4; mean FEV1 was 41 ± 13% of predicted. Mean length of stay was 4.3 ± 3.2 days. Sixty percent had ≥ 1 clinical exacerbations in the preceding year, 52% had been hospitalized at least once for a respiratory exacerbation; 61% had been hospitalized at least once; 26% were on chronic prednisone. Thirty-day readmission rate was 24%. Three variables were found to be predictive of hospitalization: Clinical exacerbations in the previous year, chronic prednisone use, and functional limitation from dyspnea predictive of hospitalization.
Conclusions: Exacerbations in the previous year, chronic prednisone use, and functional limitation from dyspnea hold promise in a scoring system used to predict 30-day re-hospitalization and could be quickly assessed from a review of hospital record or a brief interview.
Introduction
Chronic obstructive pulmonary disease (COPD) is a common disease and is a leading cause of mortality in the United States (1). Much of the cost of care in COPD involves expenses related to exacerbations of this disease (2). Hospital readmissions within 30 days in COPD are frequent – with approximately 9-20% being readmitted (3-6). Hospitals will soon be financially penalized for 30-day readmissions for COPD. Risk stratification would be useful in directing scarce medical resources toward those patients most likely to be readmitted. The objectives of our study were: 1. To evaluate predictors of 30-day hospital readmission in patients hospitalized for an exacerbation of COPD and 2. To develop a simple, clinically-useful instrument that can predict any-cause 30-day hospital readmissions in COPD patients. To this end, the final tool would have to be brief (taking < 10 minutes to complete), convenient to use and have sufficient predictive power to predict hospital readmission.
Methods
This was a prospective study, performed by means of review of medical records and patient interview. Approval for the study was obtained from the IRBs of both participating institutions. There was no extramural funding for the study.
Fifty patients admitted with acute exacerbation of COPD over a 3-month period were studied. The primary inclusion criterion was a clinical diagnosis of a COPD exacerbation resulting in hospitalization. Patients with primary diagnosis of acute exacerbation of COPD exacerbation but with concomitant diagnosis of heart failure or pneumonia were included in the analysis. Inability to effectively communicate with the investigator, including language barrier or cognitive defect was the exclusion criterion.
The hospitalist physician, after receiving verbal approval from the hospitalized COPD patient of his/her potential willingness to see an investigator for a clinical research study, was then seen by an investigator, and informed consent was obtained. Following this, an interview and review of medical records were performed to obtain demographic and disease variables. Variables (from interview or record review) included: demographics (age, gender), disease severity, all-cause and respiratory-related hospitalizations over the preceding year, outpatient treated respiratory exacerbations over the preceding year, functional status, co-morbidities, psychological status, treatment upon admission. COPD assessment test (CAT) (7), Charlson Comorbidity Index (CCI) (8) and LACE Index (9) were determined for all patients. We also measured the treating physician’s “gut feeling” of the likelihood of a 30-day readmission. The treating physician was blinded as to the specific variables we measured. (All variables tested are detailed in Appendix. Post-bronchodilator forced expiratory volume in one second (FEV1), forced vital capacity (FVC) and FEV1/FVC ratio were obtained from previous spirometry (within 3 years), if available. The patients without a historical spirometric diagnosis of COPD had spirometry before hospital discharge. Consented patients were then contacted at 30-days to determine whether they had readmissions and if so, for what cause.
General statistics are reported as means ± standard deviations (SD). Univariate logistic regression analyses were used to determine which of our tested variables predicted 30-day admission for exacerbation of COPD. Following this, multivariate forward logistic regression, incorporating variables that were predictive in univariate analyses, was utilized to determine which variables were predictive of 30-day hospitalization for COPD exacerbations.
Hospitalizations were analyzed as binary variables (yes-no). Based on the univariate analysis, two scoring systems were developed to predict readmission. The 2 scoring systems, each including three variables, significantly predicted 30-day readmissions.
The first scoring system (scoring system I) was as follows:
- MRC dyspnea. This score ranges from 0 (least) to 4 (greatest) dyspnea. Our scoring was dichotomized to 0 (MRC 0, 1, 3, or 3) or 1 (MRC 4: “too short of breath to leave the house or short of breath dressing/undressing.”
- Exacerbation history: Those with 1 or more hospitalizations for exacerbations in the preceding year were given a score of 1; those below this threshold had a score of 0.
- Chronic prednisone use prior to admission: Chronic prednisone use was defined as prednisone used on all or most days for at least three months prior to admission. Those meeting this criterion were given a score of 1, those without chronic prednisone use had a score of 0.
The second scoring system (scoring system II) was as follows:
- MRC dyspnea. This was identical to # 1 in the first scoring system.
- Exacerbation history: Those with 2 or more outpatient -treated exacerbations (some of these could result in hospitalization) in the preceding year were given a score of 1; those below this threshold had a score of 0.
- Chronic prednisone use prior to admission: This was identical to # 3 in the first scoring system.
Scores for each of the above scoring systems could, therefore, range from 0-3. The relationship between the above scores and 30-day hospital readmissions were evaluated using receiver operating characteristic (ROC) curves, which plot the true-positive rate (sensitivity) versus the false-positive rate (1-specificity).
A receiver operating characteristic (ROC) curve, plotting the true-positive rate (sensitivity) versus the false-positive rate (1-specificity) was used to characterize the relation. The ROC model was used to predict the likelihood of readmission for scoring system I and scoring system II.
Results
Of the 50 studied patients, 77% were female; mean age was 71 ± 11 years. The body mass index (BMI) was 29.65 + 9 kg/m2. Clinical characteristics of subjects are shown in Table 1.
Table 1. Clinical characteristics of studied subjects.
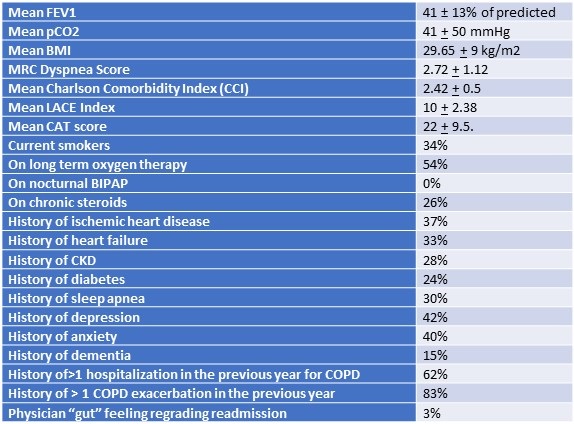
Sixty percent had Medical Research Council (MRC) dyspnea 3 or 4 (moderate to severe). Mean length of stay was 4.3 ± 3.2 days. Thirty-four percent lived alone at home.
In our study, all patients readmitted within thirty days had respiratory exacerbations of COPD as principal diagnoses (i.e., the frequency of respiratory-related and all-cause 30-day readmissions was identical). Thirty-day readmission rate for exacerbation of COPD was 24%. Of the studied parameters, the ones that did not predict rehospitalization in univariate logistic regression analyses are shown in Table 2.
Table 2. Variables that did not predict 30-day readmission.
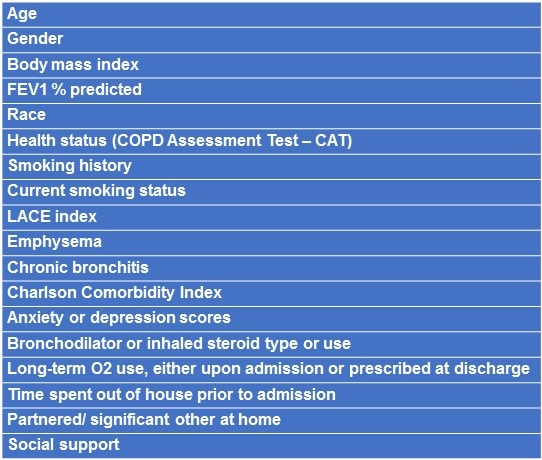
Variables that significantly predicted or tended to predict readmission included: 1) two or more clinical exacerbations (not necessarily resulting in hospitalization) in the previous year (OR 4.6, p= 0.04); 2) prednisone use (chronic or prior to admission) (OR 4.4, p< 0.04); 3) MRC = 4 (OR 2.7, p = 0.16); 4) one or more respiratory hospitalizations in the preceding year (OR 3.1, p = 0.08).
Using scoring system I, 16 patients had a score of 0; 16 had a score of 1, 14 patients had a score of 2, and 4 had a score of 3. Readmission rates for each of these categories were as follows: 13%, 19%, 29%, and 75%, respectively. Using the ROC model (Figure 1), odds ratios for readmission for- Score 0 versus 3 was 18; (2) odds ratios for readmission for score 1 versus 3 was 16 and (3) odds ratios for readmission for score 2 versus 3 was 6.7.
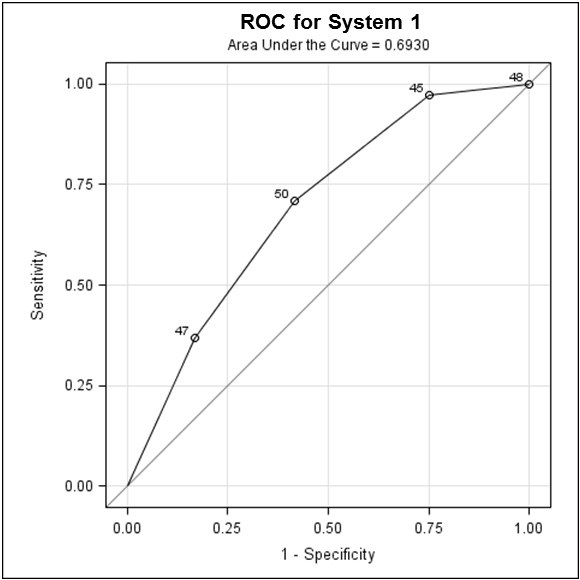
Figure 1. Receiver operating characteristic (ROC) curve for scoring system I, showing odds ratio for readmission for Score 0 versus 3, Score 1 versus 3 and Score 2 versus 3.
In scoring system II, 19 had a score of 0, 16 had a score of 1, 11 had a score of 2, and 4 had a score of 3. Readmission rates for each of these categories were as follows: 11%, 19%, 36%, and 75%, respectively. Using the ROC model (Figure 2), odds ratios for readmission for- Score 0 versus 3 was 24; (2) Score 1 versus 3 was 15 and (3) Score 2 versus 3 was 4.5.
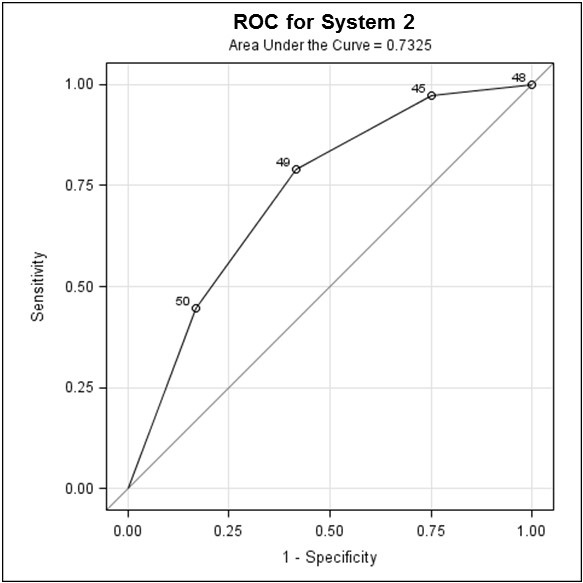
Figure 2. Receiver operating characteristic (ROC) curve for scoring system II, showing odds ratio for readmission for score 0 versus 3, score 1 versus 3 and score 2 versus 3.
In both scoring systems, the combined score of 3, with all 3 variables present, was associated with a high rate of readmission. The odds ratio was calculated for the clinical scores as it provides a valid effect measure and allows comparison of the clinical scores with regards to outcome, i.e. the readmission for COPD exacerbation, in a small study such as this.
The closer AUC is to 1, the better the predictive performance of the test, with the practical lower limit for the AUC of a predictive test being 0.5. In this study, scoring system I with an AUC of 0.69 (Figure 1) and scoring system II, with an AUC of 0.73 (Figure 2), indicate fair strength as predictors for COPD readmission.
Discussion
The purpose of our study was to create a simple scoring system that might predict 30-day readmissions in patients hospitalized with COPD exacerbations. Data regarding factors which predisposes to hospital readmissions within 30 days of discharge after hospitalization for acute exacerbations of COPD is variable and remains limited (4-6, 10,11). Our study aimed at identifying potential risk factors and evaluating probable predictors of hospital re-admission in COPD patients within a month of discharge.
In our study, three variables held promise in a scoring system used to predict re-hospitalization within 30 days: exacerbations (either clinically-treated or hospitalized), chronic prednisone use, and functional limitation from dyspnea. These three variables could be assessed within a few minutes from a review of the inpatient hospital record or from a brief interview.
Previous studies evaluating readmission risk factors in COPD up to one year have identified several variables. These include: a lower FEV1 (12- 16), reduced physical activity, functional limitation and poor health-related quality of life (2,4,17-19), need for self-care assistance, active/ passive smoking, long term supplemental O2-requirement (12,16-18), and presence of selected co-morbid conditions (20, 21).
More recent studies found low physical activity to be a significant factor (5,18). Minutes of physical activity per day in the first week following discharge was lower in those readmitted (42 + 14 minutes vs. 114 + 19 minutes, p = 0.02) (5). Ngyuen et al. (19) reported an 18% readmission rate in 4000 patients, with independent predictors of increased readmission including reduced activity, anemia, prior hospitalizations, longer lengths of stay, more comorbidities, receipt of a new oxygen prescription at discharge, use of the emergency department or observational stay before the readmission. In another retrospective study, multivariate analysis showed the following risk factors to be associated with early readmission within 30 days of discharge- male gender, history of heart failure, lung cancer, osteoporosis, and depression; no prior prescription of statin within 12 months of the index hospitalization and no prescription of short-acting bronchodilator, oral steroid and antibiotic on discharge; length of stay, <2 or >5 days and lack of follow-up visit after discharge (10). Another study found these variables to have a significant association with 30-day readmissions: age, diastolic blood pressure, COPD severity score, length of stay, pH, paCO2, FEV1< 50%, number of previous days until exacerbation (6). This study also found an increased mortality at 6 months and one year in patients readmitted within 30 days of discharge (6).
In our study, the most influential variable 30-day readmission was the history of two or more exacerbations in the preceding year (OR: 2.47, CI= 1.51-4.05, p< 0.001). This variable was also found in our study to be significantly associated with 30-day readmission, following discharge for a COPD exacerbation hospitalization.
Our study found steroid use (chronic or prior to admission) to be a significant predictor of COPD readmissions. Steroid use has been associated with a significantly increased risk of readmission in a few other studies (12,13,16,22). We hypothesize chronic prednisone use reflects instability and variability in the chronic respiratory disease or a recent exacerbation prior to the index hospitalization- hence its relatively strong relationship to re-hospitalization.
The second significant predictor in our study, exacerbations resulting in hospital admission in the preceding year, has been found to be a risk factor readmission in prior studies (6,12,13,23). Three admissions in the year preceding recruitment was found to increase risk for readmission for COPD exacerbation (12,13,23). Frequent exacerbations in the preceding year likely reflect the severity of disease in these patients. A retrospective study found no association between the number of previous hospital COPD admissions and readmission (24).
Our third significant predictor, the severity of dyspnea has also been reported in some studies to be an independent risk factor for hospital admission for an acute exacerbation of COPD. Kessler et al. (14) reported that COPD patients with a dyspnea of grade 3, 4 or 5 (defined as breathlessness with mild, minimal or limited exertion respectively), had a significant risk of hospitalization at one year but those with dyspnea of grade 2 did not. Patients with “severe dyspnea” have been found to be more likely to be readmitted to hospital in studies (15,18). Our study using the MRC rating for dyspnea and found patients with an MRC rating of 4, which is equal to the most severe grading of dyspnea in this scale. The severity of dyspnea by MRC dyspnea being a predictor for readmission in COPD indicates that the severity of the disease predisposes to exacerbations of COPD and consequent readmissions.
A systematic review of studies on risk factors for readmission for patients with COPD exacerbation found 3 predictive factors similar to our study, namely- previous hospital admission, dyspnea and oral corticosteroids (25). This review also identified other variables including use of LTOT, having low health status or poor health related quality of life and reduced routine physical activity as risk factors for admission and readmission for COPD exacerbation (25).
A scoring system similar to ours, using 3 the significant predictors of COPD readmission (chronic prednisone use, MRC dyspnea rating and prior exacerbations, either clinical or requiring hospitalizations) has not been studied in predicting the 30 day- readmission for COPD exacerbation. This scoring system was a fairly strong predictor of readmission for COPD and may serve as a useful tool in risk-stratifying patients and directing medical resources toward those patients most at risk for readmission. This is especially of relevance at the present time when hospitals will face financial penalties for 30-day readmissions for COPD.
The risk factors identified for COPD readmission in this study are not modifiable. However, if patients more at risk for readmissions can be identified based on these risk factors, more resources can be directed to these group of patients- such as closer outpatient follow-up, VNA services, inpatient and outpatient pulmonary rehabilitation, more gradual steroid taper and institution of anti-inflammatory therapy such as azithromycin.
One limiting factor of this study is the small number of patients. The scoring system generated by the study using the 3 identified predictors, though fairly predictive of readmissions for COPD exacerbations, cannot be used without corroboration. The validity of the scoring system using needs to be established in a larger group of patients. Based on the results of this study, we intend to assess these variables as part of a quality assurance study on a larger number of hospitalized COPD patients. We plan to attempt to refine the scoring system, if possible, with an emphasis on simplicity in assessing data, brevity in data collection and predictive power for 30-day and subsequent hospitalization.
Conclusions
A simple 3-point scoring system, incorporating three variables: 1) chronic prednisone use; 2) MRC dyspnea rating; and 3) prior exacerbations (either clinical or requiring hospitalizations) has a fairly high predictive value for 30 -day readmission due to COPD exacerbation. This can be easily assessed within a few minutes from a review of the inpatient hospital record or from a brief patient interview. It can serve as a useful tool in risk-stratifying patients and directing medical resources toward those patients most at risk for readmission. This scoring system using these three variables holds promise for future validation studies.
References
- Celli BR, Barnes PJ. Exacerbations of chronic obstructive pulmonary disease. Eur Respir J. 2007;29:1224-38. [CrossRef] [PubMed]
- Steer J, Gibson GJ, Bourke SC. Predicting outcomes following hospitalization for acute exacerbations of COPD. QJM. 2010;103:817-29. [CrossRef[ [PubMed]
- Johannesdottir SA. Hospitalization with acute exacerbation of chronic obstructive pulmonary disease and associated health resource utilization: a population-based Danish cohort study. J Med Econ. 2013;16:897-906. [CrossRef] [PubMed]
- Tan WC. Factors associated with outcomes of acute exacerbations of chronic obstructive pulmonary disease. COPD. 2004;1(2):225-47. [CrossRef] [PubMed]
- Sharif R, Parekh TM, Pierson KS, Kuo YF, Sharma G. Predictors of early readmission among patients 40 to 64 years of age hospitalized for chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11:685-94. [CrossRef] [PubMed]
- Guerrero M, Crisafulli E, Liapikou A, Huerta A, Gabarrus A, Chette A, Soler N, Torres A. Readmission for acute exacerbation within 30 days of discharge is associated with a subsequent increase in mortality risk in COPD patients: A long-term observational study. PLoS ONE. 2016;11:e0150737. [CrossRef] [PubMed]
- Jones PW, Harding G, Berry P, Wiklunf I, Chen WH, Kline Leady N. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34:648-54. [CrossRef] [PubMed]
- Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994:47:1245-51. [CrossRef] [PubMed]
- Walraven C, Dhalla IA, Bell C, Etchells E, Stiel IG, Zarnke K, Austin PC, Foster AJ. Derivation and validation of an Index to predict early death or unplanned readmission after discharge from hospital to community. CMAJ. 2010; 182: 551-7. [CrossRef] [PubMed]
- Garcia-Aymerich J, Monso E, Marrades RM, Escarrabill J, Felez MA, Sunyer J, Anto JM. Risk factors for hospitalization for a chronic obstructive pulmonary disease exacerbation. EFRAM study. Am J Respir Crit Care Med. 2001;164:1002-7. [CrossRef] [PubMed]
- Garcia-Aymerich J, Farrero E, Félez MA, Izquierdo J, Marrades RM, Antó JM. Risk factors of readmission to hospital for a COPD exacerbation: a prospective study. Thorax. 2003;58:100-5. [CrossRef] [PubMed]
- Gudmundsson G, Gislason T, Janson C, et al. Risk factors for rehospitalisation in COPD: role of health status, anxiety and depression. Eur Respir J. 2005;26:414–19. [CrossRef] [PubMed]
- Cao Z, Ong KC, Eng P, Tan WC, Ng TP. Frequent hospital readmissions for acute exacerbation of COPD and their associated factors. Respirology. 2006;11(2):188-95. [CrossRef] [PubMed]
- Lau AC, Yam LY, Poon E. Hospital re-admission in patients with acute exacerbation of chronic obstructive pulmonary disease. Respir Med. 2001;95:876-84. [CrossRef] [PubMed]
- Kessler R, Faller M, Fourgaut G, Mennecier B, Weitzenblum E. Predictive factors of hospitalization for acute exacerbation in a series of 64 patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159:158-64. [CrossRef] [PubMed]
- Wang Q, Bourbeau J. Outcomes and health-related quality of life following hospitalization for an acute exacerbation of COPD. Respirology. 2005;10:334-40. [CrossRef] [PubMed]
- Almargo P, Barriero B, DeEchaguen AO, Quintana S, Rodriguez CM, Heredia JL, Garau J. Risk factors for hospital re-admission in patients with chronic obstructive pulmonary disease. Respiration. 2006;73:311-7. [CrossRef] [PubMed]
- Chawla H, Bulathsinghala C, Tejada JP, Wakefield D, ZuWallack R. Physical activity as a predictor of thirty-day hospital re-admission after a discharge for a clinical exacerbation of COPD. Ann Am Thorac Soc. 2014;11:1203-9. [CrossRef] [PubMed]
- Ngyuen HQ, Chu L, Liu ILA, Lee JS, Suh D, Korotzer B, Yuen G, Desai S, Coleman KJ, Gould MK. Associations between physical activity and 30-day readmission risk in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11(5): 695-705. [CrossRef] [PubMed]
- Kessler R, Faller M, Fourgaut G, Mennecier B, Weitzenblum E. Predictive factors of hospitalization for acute exacerbation in a series of 64 patients with Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 1999;159:158–164. [CrossRef] [PubMed]
- Miravitlles M, Guerrero T, Mayordomo C, Sanchez-Agudo L, Nicolau F, Segu JL. Factors associated with increased risk of exacerbation and hospital admission in a cohort of ambulatory COPD patients: a multiple logistic regression analysis. Respiration. 2000;67:495–501. [CrossRef] [PubMed]
- Groenewegen KH, Schols AM, Wouters EF. Mortality and mortality-related factors after hospitalization for acute exacerbation of COPD. Chest. 2003; 124:459-67. [CrossRef] [PubMed]
- Connolly MJ, Lowe D, Anstey K, Hosker HSR, Pearson MG, Roberts CM. Admissions to hospital with exacerbations of chronic obstructive pulmonary disease: effect of age related factors and service organization. Thorax. 2006;61:843-8. [CrossRef] [PubMed]
- Pouw EM, Ten Velde GP, Croonen BH, Kester AD, Schols AM, Wouters EF. Early non-elective readmission for chronic obstructive pulmonary disease is associated with weight loss. Clin Nutr. 2000;19:95–99. [CrossRef] [PubMed]
- Bahadoori K, Fitzgerald JM. Risk factors of hospitalization and readmission of patients with COPD exacerbation-systematic review. Int J Chron Obstruct Pulmon Dis. 2007:2(3) 241-51. [PubMed]
Cite as: Yap V, Wilcox D, ZuWallack R, Datta D. Evaluating a scoring system for predicting thirty-day hospital readmissions for chronic obstructive pulmonary disease exacerbation. Southwest J Pulm Crit Care. 2018;16(6):350-9. doi: https://doi.org/10.13175/swjpcc054-18 PDF
 Wednesday, August 1, 2018 at 8:01AM
Wednesday, August 1, 2018 at 8:01AM 









