Jessica Hurley, MD1
Roxanne Garciaorr, MD1
Henry Luedy, MD1
Christan Jivcu, MD1
Emad Wissa, MD1
Joshua Jewell, MD1
Tonya Whiting, MD1
Richard Gerkin, MD1
Clement U. Singarajah, MD2
Richard A. Robbins, MD2
1Banner Good Samaritan Medical Center and 2Phoenix Pulmonary and Critical Care Medicine Research and Education Foundation, Phoenix, AZ
Abstract
Background
Clinical practice guidelines are developed to assist in patient care but the evidence basis for many guidelines has been called into question.
Methods
We conducted a literature review using PubMed and analyzed the overall quality of evidence and made strength of recommendation behind 8 Institute of Health Care (IHI) guidelines for prevention of central line associated blood stream infection (CLABSI). Quality of evidence was assessed by the American Thoracic Society (ATS) levels of evidence (levels I through III). We also examined data from our intensive care units (ICUs) for evidence of a correlation between guideline compliance and the development of VAP.
Results
None of the guidelines was graded at level I. Two of the guidelines were graded at level II and the remaining 6 at level III. Despite the lack of evidence, 2 of the guidelines (hand hygiene, sterile gloves) were given a strong recommendation. Chlorhexidine and use of nonfemoral sites were given a moderate recommendation. In our ICUs compliance with the use of chlorhexidine correlated with a reduction in CLABSI (p<0.02) but the remainder did not.
Conclusions
The IHI CLABSI guidelines are based on level II or III evidence. Data from our ICUs supported the use of chlorhexidine in reducing CLABSI. Until more data from well-designed controlled clinical trials become available, physicians should remain cautious when using current IHI guidelines to direct patient care decisions or as an assessment of the quality of care.
Introduction
The past three decades have seen the growth of numerous medical regulatory organizations. Many of these organizations have developed medical regulatory guidelines with over 10,000 listed under treatment/intervention in the National Guideline Clearinghouse (1). Many of these guidelines were rapidly adopted by healthcare organizations as a method to improve care. However, recent evidence suggests that many are based on opinion rather than randomized trials and most have not been shown to improve patient outcomes (2-5). We examined the IHI guidelines for prevention of CLABSI because these guidelines have been widely implemented despite what appeared to be a weak evidence basis (6).
Methods
The study was approved by the Western Institutional Review Board.
Literature Search
In each instance PubMed was searched using central line associated blood stream infection which was cross referenced with each component of the CLABSI bundle (as modified by the Veterans Administration) using the following search terms: 1. hand hygiene; 2. cap (worn by inserter); 3. mask (worn by inserter); 4. sterile gloves (worn by inserter); 5. sterile gown (worn by inserter); 6. full body drape; 7. chlorhexidine used instead of povidone iodine (betadine); and 8. femoral sites not used. Additional studies were identified from the Related Citations in PubMed and the manuscript bibliographies. Each study was assessed for appropriateness to the guideline. Studies were required to be prospective and controlled in design. Only studies that used the incidence of CLABSI as a primary outcome measure were included in assessing the quality of evidence.
Grading of level of evidence. The American Thoracic Society grading system was used to assess the underlying quality of evidence for the IHI CLABSI guidelines (7) (Table 1). A consensus was reached in each case.
Table I. Levels of Evidence
|
Level of Evidence |
Definition |
|
|
|
Level I (high) |
Evidence from well-conducted, randomized controlled trials. |
||
|
Level II (moderate)
|
Evidence from well-designed, controlled trials without randomization (including cohort, patient series, and case-control studies). Level II studies also include any large case series in which systematic analysis of disease patterns was conducted, as well as reports of data on new therapies that were not collected in a randomized fashion. |
||
|
Level III (low)
|
Evidence from case studies and expert opinion. In some instances, therapy recommendations come from antibiotic susceptibility data without clinical observations. |
||
Strength of recommendations. Seven pulmonary and critical care fellows made strength of recommendations for each guideline. This was based not only the strength of evidence but also on clinical knowledge and judgment.
Guideline Compliance and CLABSI Incidence. We also assessed our ICUs for additional evidence of the effectiveness of the individual components of the CLABSI bundle. Data were collected monthly for a period of 50 months from January, 2007 through February, 2011. This was after the Veterans Administration requirements for CLABSI reporting and compliance was instituted. Diagnosis and compliance were assessed by a single quality assurance nurse using a standardized protocol (8). Compliance with each component of the bundle was analyzed individually and expressed as a percentage. This was correlated with the incidence of CLABSI during that month expressed in line-days. Each of the following was recorded by the quality assurance nurse: (1) line days, (2) number of CLABSI and (3) the number of audits or checklists completed during central line insertion and from those checklists (4) the number of times individual bundle practices were used including femoral location. In addition to data being kept locally, data was also entered into a centralized VA website. Entry into the website required completion of a learning session and a test correctly identifying CLABSI infections in case scenarios based on CDC definitions. The program included audits because the audit tool teaches critical bundle elements and facilitates communication about bundle adherence between team members.
Statistical analysis. In some cases data were reanalyzed from original papers by Fisher’s exact test with a two-tailed comparison. For the data from our ICUs analyses were done using a Pearson correlation coefficient with a two-tailed test. Significance was defined as p<0.05.
Results
Literature Review
Numbers of articles identified by PubMed search and used for grading the level of evidence and strength of recommendation are given in Table 2. Also included are the level of evidence and the strength of the recommendation.
Table 2.
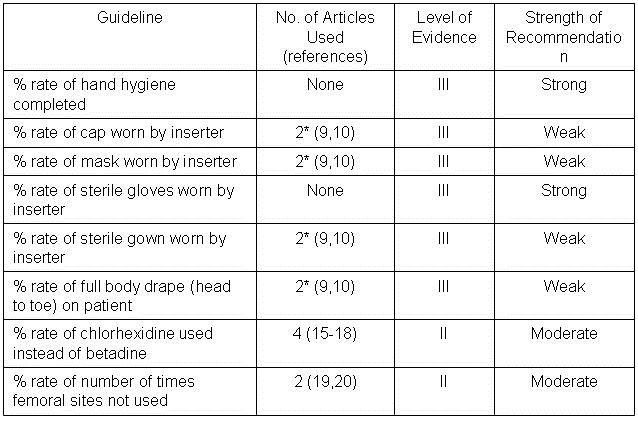 *Includes maximum barrier compared to standard barrier studies.
*Includes maximum barrier compared to standard barrier studies.
Barrier Protection. Five of the guidelines (cap worn by inserter; mask worn by inserter; sterile gown worn by inserter; and full body drape on patient) can be grouped under maximal barrier precautions as originally described by Raad et al. (9). This single site study compared 343 randomized patients to have nontunneled central catheters inserted under maximal sterile barrier precautions or control precautions (sterile gloves and small drape only). The catheters were for non-emergency venous access for chemotherapy and/or bone marrow transplantation. All patients were followed for 3 months and there were a total of four catheter infections in the test group and 12 in the control group (p= 0.03, chi-square test). However, examination of Table 3 of the manuscript revealed that 6 of the 12 in the control and 3 of the 4 in the test group had colonization rather than septicemia. Furthermore, of the remaining 7 patients only 2 developed septicemia within 30 days (both in the control group at 7 and 10 days). The remaining patients developed septicemia long after any expected benefit from barrier precautions during insertion (35-98 days). Development of septicemia this long after insertion and would seem more likely to represent contamination during handling of the catheter. Eliminating those subjects with colonization alone and septicemia after 30 days leaves 2 of 167 in the control and none of 176 in the maximum barrier precautions group. Recalculation reveals no statistically significant reduction by (p=0.24). Consistent with this reanalysis, a recent randomized, multicenter trial comparing maximum sterile barrier precautions vs. standard precautions reported by Ishikawa et al. (10) did not demonstrate a reduction in CLABSI. CLABSI developed in 5 of 211 patients with the use of maximum sterile barrier precautions compared to 6 of 213 patients with standard precautions (gloves and drape, p=1.00). Combining Raad’s revised data with Ishikawa’s data did not demonstrate a statistically significant difference between maximum and standard barrier precautions (p=0.42).
Two other studies were considered. One was a prospective but observational study by Lee et al. (11). Data from this study demonstrate a lower rate of infection with maximum sterile precautions (p=0.02) but was excluded because of the nonrandomization. Another study by Rijnders et al. (12) was a randomized study comparing maximum barrier precautions and standard precautions but with arterial lines. Maximum barrier precautions did not significantly lower the infection rate (p>0.1) but the study was excluded because it dealt with arterial rather than central venous lines.
It is also important to note that CLABSI has been directly linked to the organisms growing on the skin at the insertion site. Carrer et al. (21) found maximal sterile barrier precautions, when compared to standard care, decreased skin colonization at the insertion site for the first 48 hours (39% vs. 69%). However, within 48 hours skin colonization was no different than at 5 days. Furthermore there was no statistical difference in device colonization found between the groups (p=0.10) indicating barrier precautions did not change the rate of CLABSI. Kim and colleagues (22) found the maximal barrier precautions successfully decreased the number of gram-positive infections (p=0.05) but actually increased fungal infections (p=0.04) while having no effect on gram-negative organisms. Given the natural flora of the skin is nearly all gram-positive organisms, sole reduction of gram-positive infections alone in this study reiterates the minimal overall effect maximal barrier precautions has on CLABSI in relation to the skin organisms at the central line insertion site and suggests CLABSI often occurs in the setting of future contamination post-insertion (22).
Based on the data and a lack of clear cut rationale into a mechanism of why maximum barrier precautions should reduce CLABSI, a weak recommendation was given to each of the components of maximum barrier precautions (cap, mask, gown and drape).
Hand hygiene and sterile gloves. The effects of hand hygiene and/or sterile gloves on the development of CLABSI have not been validated in randomized controlled trials. Observational studies have demonstrated a significant decrease in the incidence of nosocomial infections with improvements in hand hygiene and use of sterile gloves (13). Decreased mortality associated with the implementation of hand hygiene dates back to Semmelweis in 1847 (14). As the simplest and least expensive means of reducing CLABSI, hand hygiene and the use of sterile gloves were strongly recommended.
Chlorhexidine. One single institution study compared 492 arterial line insertions combined with 176 central venous catheters (15). Patients were randomized to povidone iodine (227 patients), alcohol (227 patients) and chlorhexidine (224 patients) for use in insertion as well as every 48 hour cleansing of the insertion site. Seven, six and one of the patients in each group developed bacteremia respectively. Chlorhexidine use resulted in a statistically significant reduction in bacteremia if compared to the combined povidone iodine and alcohol groups. However, statistical significance was lost when analyzed on a 3x2 table or comparing povidone iodine or alcohol with chlorhexidine individually (p>0.05, all comparisons).
Mimoz et al. (16) reported a single center study in both arterial and central venous line insertions. Patients were randomly assigned to either a solution composed of 0.25% chlorhexidine gluconate, 0.025% benzalkonium chloride, and 4% benzyl alcohol or 10% povidone iodine. The same solution was used for skin disinfection from the time of catheter insertion to the time of removal of each catheter. The use of the chlorhexidine containing solution was more efficacious in preventing line related sepsis compared to povidone iodine in preventing Gram + but not Gram negative infections but there was no overall reduction of CLABSI with chlorhexidine (3 CLABSI out of 170) compared to povidone-iodine (4 CLABSI out of 145).
Another multicenter prospective, randomized, controlled trial reported by Humar et al. (17) compared 0.5% tincture of chlorhexidine to 10% povidone-iodine as cutaneous antisepsis for central venous catheter in intensive care units. Four cases of documented catheter-related bacteremia out of 193 patients were found in the chlorhexidine group compared to 5 of 181 the povidone-iodine group (p>0.05).
Combining the above studies resulted in no significant reduction with the use of chlorhexidine (8 CLABSI out of 577) compared to povidone-iodine (15 CLABSI out of 553, p=0.14).
The results of a more recent 3-year, multi-institutional, interrupted time-series design (October 2006 to September 2009), with historical control data in the pediatric intensive care unit produced differing results (18). A nested, 18-month, nonrandomized, factorial design was used to evaluate chlorhexidine scrub and chlorhexidine-impregnated sponge compliance rates. Neither was associated with a reduction in CLABSI. Due to the results only being reported in CLABSI rate per 1000 line days it was not possible to combine the data with the other studies.
One of the randomized studies showed decreased CLABSI with chlorhexidine (15). Two of the three studies showed decreased colonization as well as decreased rates of line related sepsis while the third showed decreased exit site infections (all other findings of that study did not quite make significance). All three studies were in the ICU setting, two in surgical, evaluating both central venous catheters as well as arterial lines. In this setting with likely minimal cost difference, equal to better ease of use, and smaller studies of both venous and arterial lines, the strength of recommendation was judged as moderate.
Insertion Site. One randomized study compared infections using the femoral and internal jugular sites (19). In this study the rate of CLABSI did not differ (3/313 vs. 5/313). Another study compared the femoral and subclavian sites. It also did not show a reduction with a nonfemoral site (2/127 vs. 6/100) (20). Combining the two studies did not show a significantly lower infection rate with a nonfemoral site (7/440 nonfemoral vs. 9/413 femoral, p= 0.45). Two other nonrandomized studies examined femoral compared to subclavian and internal jugular sites (23,24). One did not show a difference between the sites (23). The other, larger study showed a lower rate with non-femoral sites (24). Three other studies were considered but were found to be nonrandomized (25-27). However, complications appear higher with the femoral route including thrombosis and hematomas (28,29).
The one observational study and the higher rate of non-infectious complications resulted in the group recommending a non-femoral site when possible. The strength of this recommendation was judged as moderate.
Guideline Compliance CLABSI Incidence. In our ICUs, 1133 audits representing 11470 line-days were assessed monthly (Appendix 1). An average of 1.3 CLABSI infections/1000 line-days occurred. Correlation between the monthly compliance with each component of the CLABSI bundle with the monthly CLABSI incidence revealed only chlorhexidine use was associated with reduced CLABSI (r=-0.35, p=0.01).
Discussion This manuscript questions the validity of the CLABSI bundles as proposed by the IHI. We found that a systematic review of the literature revealed predominantly weak evidence to support these guidelines. Only one guideline (chlorhexidine) was supported by a randomized trial (15). However, data from our own ICUs showed a correlation between use of chlorhexidine and a reduction in CLABSI. The diagnosis of CLABSI is difficult, requiring clinical judgment even in the presence of objective clinical criteria (8). The difficulty in diagnosis, along with the negative consequences for failure to follow the IHI guidelines, makes before and after comparisons of the incidence of CLABSI unreliable. Therefore, we sought evidence for the effectiveness of CLABSI prevention guidelines reasoning that the better the compliance with the guidelines, the lower the incidence of CLABSI. We were unable to show that improved CLABSI guideline compliance correlated with a reduced incidence of CLABSI with the exception of use of chlorhexidine. Particularly disappointing is the data on maximum barrier precautions and reduction in CLABSI. The evidence presented in the first randomized trial was weak (9). Furthermore, when we carefully examined the data we found inclusion of catheter colonization and delays in diagnosis of over 30 days in the time from catheter insertion. It seems unlikely that contamination at the time of insertion would take over 30 days to present with sepsis. If the insertion technique was faulty (e.g., no barrier use) the infection should present within a matter of days not weeks. Many studies conflate catheter colonization with a true catheter related infection. The two entities are managed quite differently and thus need to be carefully separated (8). Reanalysis of the data eliminating the colonized patients and those who took over 30 days to present showed no reduction in CLABSI with maximum barrier protection. A more recent randomized, multicenter study would support the conclusion that there is no significant difference between maximum and standard barrier precautions (10). We could find no randomized studies of hand hygiene and gloves in the context of CLABSI prevention. However, studies in the operating room and the intensive care unit have both demonstrated that hand hygiene decreases infection (13). Both have become standards of practice. Therefore, our group felt ardently that this should be a strong recommendation. Use of povidone iodine or chlorhexidine is largely dependent on what is stocked at the time of central line insertion. We are unaware of data supporting physician preference for povidone iodine over chlorhexidine; in fact, our group almost universally prefers chlorhexidine. Although the evidence basis for chlorhexidine over povidone iodine is marginal, it seems reasonable to use chlorhexidine until the time that additional data are available, and therefore, chlorhexidine use was given a moderate recommendation. The data using non-femoral sites showed no clear cut reduction in CLABSI. The femoral site may have advantages particularly in emergency situations including ease of placement, compressibility and being distant from the head and neck during resuscitation. However, it appears to come at a higher price of both hematomas and thrombosis (19,20). Based on this our group felt a moderate recommendation was justified in nonemergent situations. Our study has several limitations. No literature review is totally comprehensive. It is possible that studies relevant to the IHI CLABSI guidelines, especially those written in a foreign language, were not identified. Second, the Phoenix VA data may be underpowered to show a small beneficial effect despite having over 11,000 line-days. Third, as with other healthcare facilities, the CLABSI guidelines at our institution were mandated and monitored. The threat of negative consequences may have compromised the objective assessment of the self-reported data, likely invalidating a before and after comparison. Fourth, correlation between guideline compliance and CLABSI incidence is not a substitute for a randomized trial. Unfortunately, the later is not possible given that guideline compliance is mandated. In the above context, this report both confirms some aspects but differs in others from a recent report by the Veterans Administration (30). In this report the VA reported data from all ICUs and found a reduction in CLABSI and an increase in compliance from 2006-9. Although the database is much larger than the data in this report from a single institution, it suffers from the same weaknesses as our data. Not reported are the death or morbidity rates from CLABSI. Interestingly, CLABSI rates were much lower in smaller hospitals (level 4). Whether these hospitals had increased compliance was not reported, but these smaller hospitals are known to have higher all cause mortality, surgical mortality and surgical morbidity (31). Guidelines have taken on the aura of law which is substituted for clinical judgment. For example, a nurse practitioner attempted to prevent a senior critical care physician in one of our facilities from inserting a central line because the physician was not wearing a cap. However, since the patient had no line access and was in extremis, the physician decided to proceed. Other examples are the decisions to place a femoral catheter during emergencies, when other venous access is unavailable, when a pneumothorax might be catastrophic, or when major bleeding is a risk (the femoral vein is compressible). Clinical judgment might weigh the risks of internal jugular or subclavian insertions compared to the femoral vein and conclude that the femoral site might be the best choice for the patient. It is unclear why the IHI guidelines have received such wide acceptance given their weak evidence basis. Agencies involved in guideline writing should show restraint in guideline formulation based on opinion or weak or conflicting evidence. Only those interventions based on strong evidence which can make a real difference to patients should be designated as guidelines. Acknowledgements The authors acknowledge Janice Allen, MSN, RN who collected the CLABSI data reported from the Phoenix VA. References Refernce as: Hurley J, Garciaorr R, Luedy H, Jivcu C, Wissa E, Jewell J, Whiting T, Gerkin R, Singarajah CU, Robbins RA. Correlation of compliance with central line associated blood stream infection guidelines and outcomes: a review of the evidence. Southwest J Pulm Crit Care 2012;4:163-73. (Click here for a PDF version of manuscript)