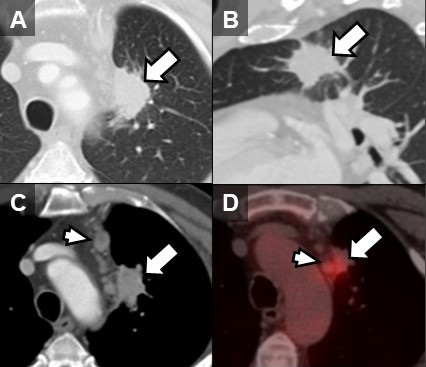
Figure 1. Unenhanced lung window chest CT images in the axial (A) and sagittal (B) planes show a solid, non-calcified irregular left upper lobe mass (arrow) with spiculated margins. The nodule demonstrates enhancement on soft tissue windows (C) with associated mediastinal adenopathy (arrowhead). The mass and adenopathy are FDG-avid on axial fused PET-CT image (D).
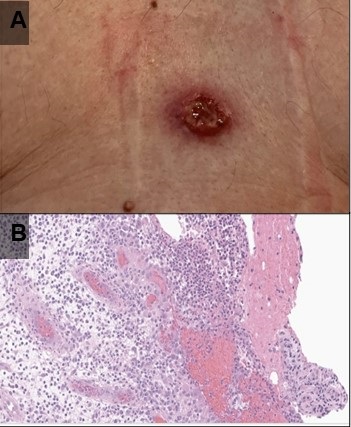
Figure 2. (A) Photograph of one of the patient’s skin lesions. (B) Hematoxylin and Eosin stained low-power pathological image of a biopsy specimen from a skin lesion demonstrates dense mixed neutrophilic dermal inflammation. Extensive infectious and neoplastic workup was negative. The histopathologic diagnosis was consistent with pyoderma gangrenosum.
A 70-year-old man presented with persistent cough productive of clear sputum which had persisted approximately 12 months after COVID-19 infection. The patient reported a more recent history of night sweats and had also recently developed what he described as “blisters” on his chest wall and right shoulder starting 4 weeks prior to presentation that “opened up” giving off a bloody discharge. The patient had been treated with trimethoprim-sulfamethoxazole and doxycycline without improvement and reported a 10-pound weight loss over the past several months. The patient was a never-smoker with no significant travel history and a past medical history of asthma, GERD, gout, and chronic rhinitis. He had no history of autoimmune/inflammatory diseases or malignancy.
Vital signs and physical exam were normal, except for a 1 cm open wound in the center of the patient’s chest [Figure 2A]. A chest CT performed as part of the patient’s workup demonstrated a spiculated mass in the left upper lobe with adjacent mediastinal adenopathy [Figure 1A-C]. This prompted an FDG PET-CT, which demonstrated some increased uptake in the mass and adjacent lymph nodes [Figure 1D]. The mass was biopsied via bronchoscopy, pathology was nondiagnostic with rare groups of benign-appearing bronchial epithelial cells and blood. The skin lesion was biopsied next demonstrating dense mixed neutrophilic dermal inflammation [Figure 2B]. The diagnosis of pyoderma gangrenosum was made and the patient was treated with NSAIDs and a systemic glucocorticoid (40 mg/day, tapered over 10 weeks).steroid taper, The pulmonary mass , mediastinal lymph nodes and skin lesions all resolved over time.
Pyoderma gangrenous (PG) is a misnomer in every sense as it is neither infectious nor gangrenous. It is a rare (3-10 cases/million/year) disorder of skin characterized by neutrophilic dermatosis which usually presents as a with inflammatory and ulcerative disorder of the skin lesions and is usually a diagnosis of exclusion (1). PG has no pathognomonic clinical or histological findings. Majority of the cases have an underlying systemic disease, commonly inflammatory bowel disease (41%), inflammatory arthritis (20.5%) and oncologic or hematologic disorders (17.2%). While it can in any age group including children, the peak age of onset is 40-60 years. There is a slight female preponderance (2). The most common presentation is inflammatory papule or pustule that progress to a painful ulcer with violaceous undermined borders and a purulent base. The lesions commonly occur in surgical wounds within 2 weeks of surgery, a phenomenon known as pathergy, and often lead to wound dehiscence (3). The lesions may also be peristomal in patients with IBD. Extracutaneous lesions have been reported in liver, intestine, spleen, cornea, bones, muscles, CNS and rarely, in the lungs (4-6).
There have been <50 cases of pulmonary PG ever described in literature (7,8). The patients may present with non-specific symptoms of cough, dyspnea, fever, weight-loss, malaise and occasionally hemoptysis. Chest imaging may show cavitary infiltrates. The diagnosis is established by cutaneous or extracutaneous lesion biopsy of the ulcer edge showing neutrophilic infiltrate. Extensive testing should be performed , extensive testing to rule out alternative causes including infection, and malignancy, in setting of underlying inflammatory bowel disease or inflammatory arthritisautoimmune and inflammatory conditions associated with PG. Presence of pathergy and response to anti-inflammatory therapy also support the diagnosis (9). Treatment includes systemic or intralesional glucocorticoids and/or calcineurin inhibitors (3). Use of TNF alpha inhibitor, infliximab and anti-neutrophil antimicrobial dapsone has also been described in case reports (10). Most patients achieve remission within 6 months to 3 years.
Umesh Goswami MD1, Michael Gotway MD2, Carlos Rojas MD2, Prasad Panse MD2, Kris Cummings MD2, Eric Jensen MD2, Kenneth Sakata, MD1 and Clinton Jokerst MD2
Division of Pulmonology1 and Department of Radiology2
Mayo Clinic Arizona, Scottsdale, AZ USA
References
- Ruocco E, Sangiuliano S, Gravina AG, Miranda A, Nicoletti G. Pyoderma gangrenosum: an updated review. J Eur Acad Dermatol Venereol. 2009 Sep;23(9):1008-17. [CrossRef] [PubMed]
- Ashchyan HJ, Butler DC, Nelson CA, et al. The Association of Age With Clinical Presentation and Comorbidities of Pyoderma Gangrenosum. JAMA Dermatol. 2018 Apr 1;154(4):409-413. [CrossRef] [PubMed]
- Binus AM, Qureshi AA, Li VW, Winterfield LS. Pyoderma gangrenosum: a retrospective review of patient characteristics, comorbidities and therapy in 103 patients. Br J Dermatol. 2011 Dec;165(6):1244-50. [CrossRef] [PubMed]
- Vadillo M, Jucgla A, Podzamczer D, Rufi G, Domingo A. Pyoderma gangrenosum with liver, spleen and bone involvement in a patient with chronic myelomonocytic leukaemia. Br J Dermatol. 1999 Sep;141(3):541-3. [CrossRef] [PubMed]
- Scherlinger M, Guillet S, Doutre MS, Beylot-Barry M, Pham-Ledard A. Pyoderma gangrenosum with extensive pulmonary involvement. J Eur Acad Dermatol Venereol. 2017 Apr;31(4):e214-e216. [CrossRef] [PubMed]
- Abdelrazeq AS, Lund JN, Leveson SH. Pouchitis-associated pyoderma gangrenosum following restorative proctocolectomy for ulcerative colitis. Eur J Gastroenterol Hepatol. 2004 Oct;16(10):1057-8. [CrossRef] [PubMed]
- Gade M, Studstrup F, Andersen AK, Hilberg O, Fogh C, Bendstrup E. Pulmonary manifestations of pyoderma gangrenosum: 2 cases and a review of the literature. Respir Med. 2015 Apr;109(4):443-50. [CrossRef] [PubMed]
- Sakata KK, Penupolu S, Colby TV, Gotway MB, Wesselius LJ. Pulmonary pyoderma gangrenosum without cutaneous manifestations. Clin Respir J. 2016 Jul;10(4):508-11. [CrossRef] [PubMed]
- Maverakis E, Ma C, Shinkai K, et al. Diagnostic Criteria of Ulcerative Pyoderma Gangrenosum: A Delphi Consensus of International Experts. JAMA Dermatol. 2018 Apr 1;154(4):461-466. [CrossRef] [PubMed]
- Teasley LA, Foster CS, Baltatzis S. Sclerokeratitis and facial skin lesions: a case report of pyoderma gangrenosum and its response to dapsone therapy. Cornea. 2007 Feb;26(2):215-9. [CrossRef] [PubMed]
Cite as: Goswami U, Gotway M, Rojas C, Panse P, Cummings K, Jensen E, Sakata K, Jokerst C. July 2022 Medical Image of the Month: Pulmonary Nodule in the Setting of Pyoderma Gangrenosum (PG). Southwest J Pulm Crit Care Sleep. 2022:25(1):4-6. doi: https://doi.org/10.13175/swjpccs029-22 PDF