Matthew D Rockstrom, MD1
Jonathan D Rice, MD1,2
Tomio Tran, MD3
Anna Neumeier, MD1,4
1Department of Medicine, University of Colorado School of Medicine, Aurora, CO USA
2Department of Medicine, Division of Gastroenterology and Hepatology, University of Colorado School of Medicine, Aurora, CO USA
3Department of Medicine, Division of Cardiology, University of Washington, Seattle, WA USA
4Department of Medicine, Division of Pulmonary Sciences and Critical Care, Denver Health and Hospital Authority, Denver, CO USA
Abstract
Acute liver failure (ALF) is characterized by acute liver injury, coagulopathy, and altered mental status. Acetaminophen overdose contributes to almost half the cases of ALF in the United States. In the era of liver transplantation, mortality associated with this condition has improved dramatically. However, many patients are not transplant candidates including many who present with overt suicide attempt from acetaminophen overdose. High volume plasma exchange (HVP) is a novel application of plasma exchange. Prior research has shown that HVP can correct the pathophysiologic derangements underlying ALF. A randomized control trial demonstrated improved transplant-free survival when HVP was added to standard medical therapy. In this case, we examine a patient who presented to the intensive care unit with ALF caused by intentional acetaminophen overdose. She was denied transplant due to overt suicide attempt, was treated with HVP, and made a rapid recovery, eventually discharged to inpatient psychiatry and then home.
Abbreviations: ALF: acute liver failure: CVVH: continuous veno-venous hemodialysis; DAMPs: damage associated molecular patterns; FFP: fresh frozen plasma; HVP: high volume plasma exchange; MODS: multisystem organ dysfunction; NAC: N-acetyl cysteine; NNT: Number needed to treat; SIRS: systemic inflammatory response syndrome; SMT: standard medical therapy; TNF-α: tumor necrosis factor alpha
Introduction
Acute liver failure (ALF) is a rare, life-threatening condition. Although survival has improved in the transplant era, mortality remains high without transplantation. Here we discuss a novel therapy for ALF patients which may provide improved transplant-free mortality.
Case Report
A 21-year-old woman arrived by ambulance, found to be obtunded and hypotensive in the field, with an empty bottle of acetaminophen and a suicide note. She had a history of depression, infrequent alcohol and marijuana use, and was otherwise healthy.
Upon presentation, she was afebrile (temperature 36.5°C), tachycardic (heart rate 155 beats-per-minute) and hypotensive requiring norepinephrine of 0.1 μg/kg/min to maintain mean arterial blood pressure above 65. Due to grade IV encephalopathy, she was intubated. Admission lab work is shown below (Table 1). Viral hepatitis and HIV serologies were negative and ultrasound demonstrated patent vasculature and normal liver parenchyma.
Table 1: Lab work on admission, hospital day 2, and following high-volume plasma exchange therapy.
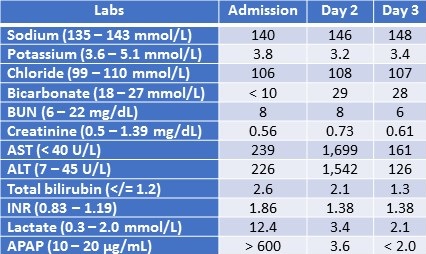
BUN: blood urea nitrogen, AST: aspartate aminotransferase; ALT: alanine aminotransferase; INR: international normalized ratio; APAP: N-acetyl-para-aminophenol
N-acetyl cysteine (NAC) was administered and transplant evaluation was obtained. Despite meeting King’s College Criterion for transplantation, she was declined due to presentation for suicide attempt. She was managed supportively with vasopressors, continuous veno-venous hemodialysis (CVVH), and high-volume plasma exchange (HVP) at a rate of 8 liters of fresh frozen plasma (FFP) daily, receiving 24 liters total. After initiation of HVP, vasopressors were immediately weaned. The following day, her encephalopathy improved, and she followed simple commands. CVVH was discontinued on hospital day 4. She was extubated on hospital day 6 and was eventually discharged home.
Clinical Discussion
ALF is a life-threatening syndrome characterized by acute liver injury, encephalopathy, and coagulopathy. In the United States, the most common etiology is acetaminophen overdose, accounting for ~46% of cases (1). Standard medical therapy (SMT) is supportive, treating the underlying etiology and mitigating manifestations of multisystem organ dysfunction (MODS). The advent of transplantation dramatically improved the mortality associated with ALF but the benefit of transplant must be balanced with high-risk surgery, lifelong immunosuppression, and organ scarcity (2). Given these risks, patients undergo evaluation including psychologic evaluation which commonly excludes patients presenting with intentional acetaminophen overdose. Without transplantation, mortality for these patients remains high.
The pathophysiology of ALF is not entirely understood but is largely driven by hepatic necrosis leading to hepatic metabolic dysfunction and release of intracellular contents. Intracellular damage associated molecular pattern (DAMPs) and Kupffer cell activation trigger the release of pro-inflammatory cytokines like tumor necrosis factor alpha (TNF-α), which result in systemic inflammatory response syndrome (SIRS) and vasodilation (3,4). Subsequent hepatic metabolic dysfunction is manifested by hyperbilirubinemia, hyperammonemia, coagulopathy, and hypoglycemia.
High volume plasma exchange (HVP) has shown promise as a new modality of treatment for patients with ALF. A new implementation of plasma-exchange therapy, patient plasma is exchanged with donor FFP. In one prospective, randomized control trial by Larsen et al, 15% of ideal body weight of FFP was exchanged daily for three days in addition to SMT. HVP plus SMT improved survival to discharge when compared to SMT alone (58.7 % versus 47.8%, respectively; number needed to treat (NNT) 9.2) (5). HVP plus SMT has been shown to reverse clinical parameters associated with ALF including INR, bilirubin, vasopressor requirements, reliance on renal replacement, hepatic encephalopathy (5-7). HVP was also shown to significantly attenuate DAMPs, including IL-6 and TNF-α, indicating an ability to attenuate the biochemical nidus of MODS (6,7). A systematic review of HVP found evidence of mortality benefit in HVP for both ALF and acute on chronic liver failure, though Larsen et al remains the only randomized prospective trial. Subsequently, HVP has become a level I, grade 1 recommendation in European guidelines for ALF (6).
There are limitations associated with HVP including utilization of FFP, concerns for precipitation volume overload, and worsening cerebral edema. Additionally, there is no clear optimal regimen for dose and duration of HVP. In a recent randomized control trial by Maiwall et al, standard volume plasma exchange was shown to improve transplant free survival using only 1.5 to 2 times calculated patient plasma volume (4).
Conclusion
In this case, a 21-year-old patient presented with ALF following acetaminophen overdose. Despite qualifying for transplantation, she was denied due to presentation for suicide attempt. She was treated with standard medical therapy and HVP and had rapid improvement in hemodynamics and mentation. While it is impossible to quantify the degree to which HVP contributed to her recovery, her clinical improvement was dramatic despite presentation with severe disease. HVP has been shown to reverse the pathophysiologic hallmarks of ALF, improve transplant-free mortality, and is now a level I recommendation according to European guidelines. More trials are necessary to determine the optimal dose and duration of this life saving modality.
References
- Lee WM. Etiologies of acute liver failure. Semin Liver Dis. 2008 May;28(2):142-52. [CrossRef] [PubMed]
- Lee WM, Squires RH Jr, Nyberg SL, Doo E, Hoofnagle JH. Acute liver failure: Summary of a workshop. Hepatology. 2008 Apr;47(4):1401-15. [CrossRef] [PubMed]
- Chung RT, Stravitz RT, Fontana RJ, Schiodt FV, Mehal WZ, Reddy KR, Lee WM. Pathogenesis of liver injury in acute liver failure. Gastroenterology. 2012 Sep;143(3):e1-e7. [CrossRef] [PubMed]
- Maiwall R, Bajpai M, Singh A, Agarwal T, Kumar G, Bharadwaj A, Nautiyal N, Tevethia H, Jagdish RK, Vijayaraghavan R, Choudhury A, Mathur RP, Hidam A, Pati NT, Sharma MK, Kumar A, Sarin SK. Standard-Volume Plasma Exchange Improves Outcomes in Patients With Acute Liver Failure: A Randomized Controlled Trial. Clin Gastroenterol Hepatol. 2021 Jan 29:S1542-3565(21)00086-0. [CrossRef] [PubMed]
- Larsen FS, Schmidt LE, Bernsmeier C, et al. High-volume plasma exchange in patients with acute liver failure: An open randomised controlled trial. J Hepatol. 2016 Jan;64(1):69-78. [CrossRef] [PubMed]
- Tan EX, Wang MX, Pang J, Lee GH. Plasma exchange in patients with acute and acute-on-chronic liver failure: A systematic review. World J Gastroenterol. 2020 Jan 14;26(2):219-245. [CrossRef] [PubMed]
- Larsen FS, Ejlersen E, Hansen BA, Mogensen T, Tygstrup N, Secher NH. Systemic vascular resistance during high-volume plasmapheresis in patients with fulminant hepatic failure: relationship with oxygen consumption. Eur J Gastroenterol Hepatol. 1995 Sep;7(9):887-92. [PubMed]
Cite as: Rockstrom MD, Rice JD, Tran T, Neumeier A. High Volume Plasma Exchange in Acute Liver Failure: A Brief Review. Southwest J Pulm Crit Care. 2021;22(5):102-5. doi: https://doi.org/10.13175/swjpcc009-21 PDF