Ronald Ferrer Espinosa DO1
Abdirahman Hussein MD2
Matthew Sehring DO1
Mohamad Rachid MD1
Ryan Dunn MD1
Deepak Taneja MD1
Department of Pulmonary and Critical Care Medicine1
Department of Internal Medicine2
University of Illinois College of Medicine at Peoria
Peoria, Illinois
Abstract
Introduction: Since their introduction, electronic cigarette use has increased and was even proposed as an alternative to traditional tobacco use. Recently, a series of patients with acute respiratory failure due electronic cigarette, or vaping, associated lung injury (EVALI) in 2019 has been described which has largely been attributed to tetrahydrocannabinol (THC) containing vaporizer itself, as well as vitamin E acetate. Several case series have been published regarding the acute presentation, diagnosis and management. In addition to diagnosis and management of EVALI, we sought to describe potential long-term effects of lung parenchyma in these patients.
Methods: A retrospective review was performed on 16 patients with clinically diagnosed EVALI at OSF St Francis Medical Center between August 01 2019 and February 1 2020. Relevant demographic and clinical data were collected in patients diagnosed with EVALI.
Results: Of the 16 patients in the study the median age (IQR) age was 25.25 (20-29) and 94% were male. The predominant presenting symptoms were dyspnea (94%), cough (56%), nausea 63%), vomiting (63%), abdominal pain (50%), diarrhea (50%), and fever (63%). 2 (13%) patients required endotracheal intubation. Common features of computerized tomography (CT) scan were bilateral diffuse ground glass opacity (93%), septal thickening (53%), and subpleural sparing (47%). Bronchoalveolar lavage (BAL) was obtained in 3 patients and all demonstrated neutrophil predominance of 69% (56-90). One BAL was significant for hemosiderin laden macrophages. Post hospital follow up pulmonary function tests were obtained in 3 and 2 of these were significant for obstructive lung disease.
Conclusions: In this case series of patients diagnosed with vaping associated lung injury, obstructive lung disease may be seen on pulmonary function testing and surveillance of these patients should occur regardless of duration.
Keywords: CT scan, EVALI, bronchoalveolar lavage, electronic cigarette, pulmonary function testing, respiratory failure, tetrahydrocannabinol, vaping, vaping associated lung injury, vitamin E,
Introduction
The first cases of vaping associated lung injury (EVALI) were reported in Wisconsin and Illinois in the summer of 2019 which reached its peak in the fall of 2019 (1). This sudden epidemic of respiratory failure in patients who used tetrahydrocannabinol (THC) containing vaporizing devices lead the Food and Drug Administration (FDA), Center for Disease Control (CDC) and local public health departments to initiate investigations and research into the causative mechanisms of this disease. Currently, it is postulated that vitamin E acetate plays a role in the pathogenesis of VALI, as this substance was found in samples of vaping cartridges and in the bronchoalveolar fluid of patients with the disease (2,3). These pathological pathways are still being elucidated. Little is known about the long-term damage to the respiratory system in patients with EVALI. In this study, we sought to describe the diagnostic commonalities in patients with EVALI and describe potential long-term complications.
Methods
The study was a retrospective cohort analysis. Institutional Review Board (IRB) approval (1593746-1) was obtained through the University of Illinois College of Medicine at Peoria IRB. Data was collected for consecutive patients over 18 years of age who were diagnosed with EVALI between the dates of August 1, 2019 and February 1, 2020. The diagnosis of EVALI was consistent with the outbreak case surveillance definition. A confirmed case required the following to satisfy criteria: e-cigarette or dabbing in 90 days before symptom onset, pulmonary infiltrate, absence of infection, and no evidence of alternative plausible causes. A presumptive case definition included the above definition except for possibility of another cause of the patient’s symptoms such as infection. Data were extracted from the electronic medical record. The recorded data included the following: age, gender, co-morbidities, tobacco and electronic cigarette use history, need for endotracheal intubation, symptoms on presentation to the emergency department, computerized tomography findings, pulmonary function test values, bronchoalveolar lavage fluid studies, and discharge treatment plans. Obstructive lung disease is based on the American Thoracic Society/European Respiratory Society criteria that recommends the fifth percentile of the distribution in a population of healthy lifelong nonsmokers as the lower limit of normal. One patient described in this study has been previously described (4). Continuous variables are presented at median and interquartile range (IQR) with 95% CI. Categorical variables are described as number of patients (percentage).
Results
From August 1, 2019 to February 1, 2020, a total of 16 patients with either confirmed or presumptive vaping associated lung injury were reviewed. Table 1 shows the demographic data obtained from these patients.
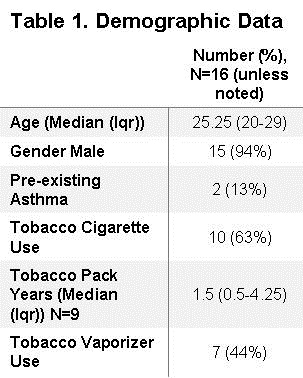
The median age was 25.25 (IQR, 20-29) and the majority of patients were male 94% (n=15). Only 13% (n=2) of patients had previously diagnosed lung conditions, both of which were asthma. Of the reported THC brands, Dank© was the most commonly reported occurring in 25% (n=4) of patients (Table 2).
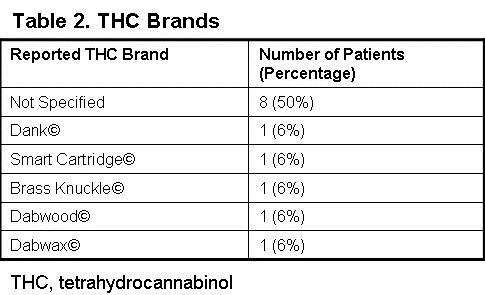
However, 50% (n=8) of THC products were not clearly stated in the patient’s medical record. Tobacco cigarette and tobacco electronic cigarette use were also documented, occurring in 63% (n=10) and 44% (n=7) of patients, respectively. Of those reporting tobacco use, the median pack years was 1.5 (IQR, 0.5-4.25). 7 patients reported no prior tobacco use. 13% (n=2) of patients required endotracheal intubation
Patients' symptoms are summarized in Table 3.
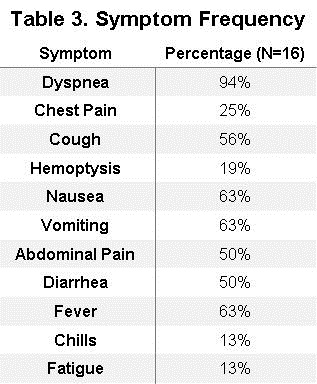
Any respiratory symptom occurred in 94% (n=15) of patients which included dyspnea 94% (n=15), cough 56% (n=9), chest pain 25% (n=4), and hemoptysis 19% (n=3). Abdominal symptoms were common and occurred in 75% (n=12) of patients. The most common gastrointestinal symptom were both nausea and vomiting 63% (n=10). These were followed by abdominal pain and diarrhea 50% (n=8). Fever was the most common constitutional symptom occurring in 63% (n=10) of patients. Less common constitutional symptoms included fatigue and chills both of which occurred in 13% (n=2) of patients.
Chest computerized tomography (CT) scans were available in 94% (n=15) of patients. The findings are summarized in Table 4.

The most common radiographic feature was bilateral diffuse ground glass opacity (GGO), which occurred in 93% (n=14) of patients. Septal thickening and subpleural sparing were the next most common radiographic findings, occurring in 53% (n=8) and 47% (n=7) patients, respectively. Less common features that were described on chest CT scan were centrilobular nodular consolidations occurring in 27% (n=4) and reverse haloing occurring in 7% (n=1, Figure 1).
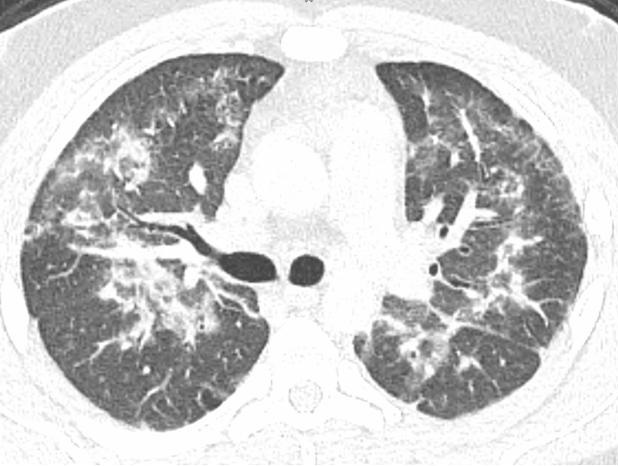
Figure 1. Reverse halo sign in a patient with EVALI.
Three patients diagnosed with EVALI underwent bronchoscopy with bronchoalveolar lavage which are summarized in table 5.
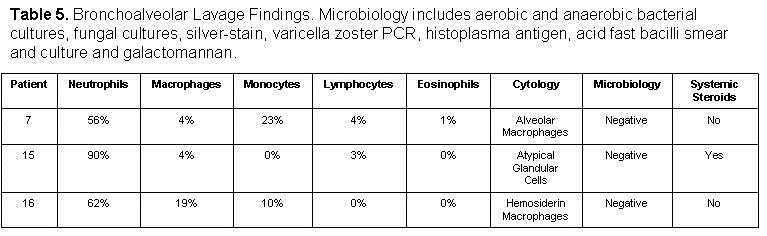
Bronchoscopy was performed when the outbreak case definition was not met or there was a clinical concern for a secondary pathological process. The “typical” bronchoalveolar lavage (BAL) differential was neutrophilic predominant (56%-90%) with elevated macrophage and/or monocyte counts (4-19%, 0-23%). The lymphocyte count was typically low (0-4%) as well as the eosinophil count (0-1%). All the microbiologic data from these lavage samples were negative and included the following tests: aerobic and anaerobic bacterial cultures, fungal cultures, silver stain, acid fast bacilli smear and culture, varicella zoster polymerase chain reaction (PCR), histoplasma antigen and galactomannan. The results of cytology were different in all three BAL samples and were significant fore alveolar macrophages, atypical glandular cells and hemosiderin laden macrophages. At the time of bronchoscopy, only 1 or 3 patients was on or received systemic steroids therapy.
A few complications occurred in our cohort which included pneumothorax, pneumorrhachis, pulmonary embolism and Takutsubo cardiomyopathy. The case of Takusubo cardiomyopathy has been reported in the literature (4). The rate of pneumothorax was 13% (n=2). The pneumorrhachis 6% (n=1) occurred in a patient with pneumothoraces. Pulmonary embolism was only seen in 1 patient.
The discharge treatment course involved mainly systemic corticosteroids which were given in 81% (n=13) of patients. Antibiotics were continued at discharge in a minority of patients 38% (n=6). The majority of patients (94%) recovered and were asymptomatic at a later clinic visit. One patient remained symptomatic with persistent dyspnea.
Full pulmonary function tests were obtained in 3 patients (Table 6).
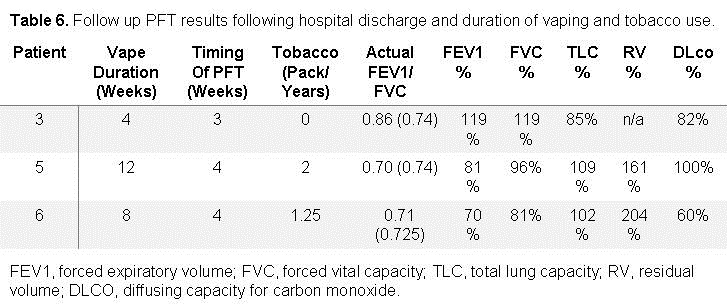
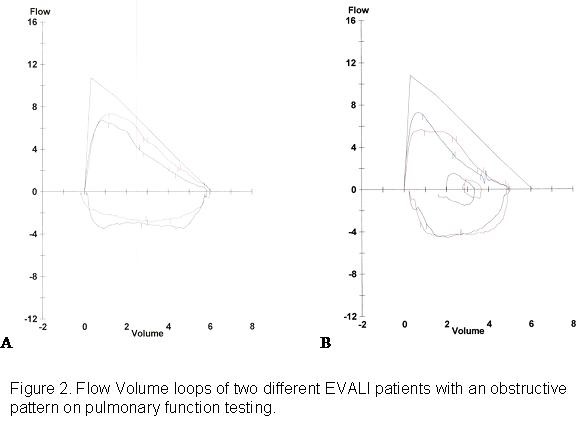
The timing of the pulmonary function tests occurred 3-4 weeks post hospital discharge. THC vape duration for these patients ranged from 4-12 weeks and tobacco pack years ranged from 0-2. Forced expiratory volume in 1 second to forced vital capacity (FEV1/FVC) ratios were 70%, 71%, and 86%. FEV1% ranged from 70 to 119%. FVC ranged from 81% to 119%. The total lung capacity in these three patients ranged from 85% to 109%. Diffusing capacity of carbon monoxide (DLCO) ranged from 60% to 100%. The flow volume loops of two patients demonstrate coving of the expiratory limb (Figure 2).
Pre-EVALI pulmonary function testing was not available for review in any of the patients.
Discussion
In this case series of patients with vaping associated lung injury of 16 patients in Central Illinois from August 1 2019 through February 1, 2020 the majority were younger men with respiratory and gastrointestinal symptoms. The symptomatology was consistent with previously published data (1,5,6,7). The exact pathophysiologic mechanisms implicated in vaping associated lung injury are currently still under investigation, but vitamin E acetate has recently been implicated possibly through the transition of tocopherols induced transition of phosphatidiylcholines from gel to liquid crystalline, causing surfactant dysfunction (2). The same authors proposed an alternative mechanism whereby ketene, created while heating e-cigarette products, causes direct lung irritation. However, many branded vaping products are not commercially produced and the heterogenous ingredients such as propylene glycol and other flavoring ingredients in these products may reflect its informal creation, which may influence the development of different disease mechanisms, clinical phenotypes and imaging findings (8,9).
A growing body of EVALI cases demonstrate predominant features on computerized tomography which include basilar predominant centrilobular nodular ground glass opacities and ground glass opacities with subpleural sparing. An initial study proposed four imaging patterns which included acute eosinophilic pneumonia, diffuse alveolar damage, organizing pneumonia and lipoid pneumonia and predicted the dominant CT scan findings (10). Later in a small case series of pediatric patients, centrilobular ground glass opacities were present in 92% and ground glass opacities with subpleural sparing were present in 75% of patients (11). The importance of recognizing these patterns is essential, especially in the adolescent and young adult populations where disclosure of medical history may prove to be difficult. Our findings support the cases in literature, with 93% of patients having bilateral diffuse basilar predominant GGO (93%), some associated with subpleural sparing (47%) and to a lesser degree centrilobular nodular consolidation (27%).
The role of bronchoscopy in patients with vaping associated lung injury has waned with as the characterization of clinical and radiographic findings has matured. Importantly, there is no finding on bronchoscopy that is specific for diagnosis of EVALI. Recently, the role of bronchoscopy with bronchoalveolar lavage (BAL) has been suggested to be performed in cases with a high pretest probability of EVALI with atypical features that cannot be attributed to vaping (5). Lipid laden macrophages (LLM) are a non-specific finding in many different illnesses such as infection, aspiration, drug reactions, lipoid pneumonia, pulmonary alveolar proteinosis and autoimmune disorders, but the absence of LLM, argued by Aberegg, would be an atypical finding in EVALI (5,12). Our BAL fluid differentials are consistent with the published data, with elevations in neutrophils and/or monocytes and macrophages, with scant lymphocytes and eosinophils. In our institution, staining for lipid laden macrophages was not routinely performed. However, in one of our cytologic BAL samples hemosiderin macrophages were identified. The inconsistent cytologic findings on BAL in our series supports the notion of heterogenous underlying pathophysiologic mechanisms involved in EVALI.
The long-term effects of EVALI on lung parenchyma are unknown. Prior studies evaluating the effects of electronic cigarette use prior to the EVALI epidemic have suggested airway hyper responsiveness and an obstructive pattern on spirometry. In mice, it has been previously demonstrated that aerosolized nicotine induced airway hyperreactivity (13). A human study of 30 electronic cigarette users with at least 6 months of use compared with matched controls, demonstrated PFTs consistent with peripheral obstruction (14). A different study comparing vaping asthmatics versus healthy controls demonstrated acute declines in the FEV1/FVC ratio (15). Additionally, a few recent reports of pulmonary function testing have been documented in patients diagnosed with EVALI in late 2019 and early 2020. One study of intubated adults reported one follow-up PFT that was significant for only a low DLCO (16). A case series of pediatric patients describes two patients with 6 week follow up PFT’s that were significant for obstructive lung disease, and one of these patients had a low DLCO (11). Our study contributes 3 full PFT’s in adult patients diagnosed with EVALI performed at either 3 or 4 weeks following hospital discharge. Two of these PFT’s demonstrated obstructive lung disease, of which one had a low DLCO. The duration of vaping in these two abnormal cases were 8 and 12 weeks, and the pack years of tobacco use were 2 and 1.25 years. This small data set suggests that patients who participate in electronic cigarette use, or vaping, may be at higher risk for developing obstructive lung disease regardless of the duration of use. It is also important to take into account that a fixed FEV1/FVC ratio may underestimate young patients with obstructive lung disease (17). Using the lower limit of normal in this younger patient population is likely to be more sensitive in detecting obstructive physiology. It is plausible that vaping may cause an accelerated obstructive lung pattern due to the many potential pathways for lung injury. Full pulmonary function testing in any patient who was diagnosed with EVALI or continues to use electronic cigarettes, or vaping, products should be considered.
This study has several limitations. First, this was a retrospective study of patients at a single center in one geographic location. Second, our sample size was relatively small. Third, our clinical follow up rate was only 50%, of which only 37% completed PFT’s.
Conclusions
In our case series of 16 patients, predominantly male, diagnosed with EVALI there was a high incidence of gastrointestinal symptoms on presentation and follow up pulmonary function tests suggested there may be an increased risk for obstructive lung disease. Avoidance of vaping products, especially “Dank” and other similarly formulated products, is strongly recommended.
References
- Layden JE, Ghinai I, Pray I, et al. Pulmonary Illness Related to E-Cigarette Use in Illinois and Wisconsin - Final Report. N Engl J Med. 2020 Mar 5;382(10):903-916. [CrossRef] [PubMed]
- Blount BC, Karwowski MP, Shields PG, et al. Vitamin E Acetate in Bronchoalveolar-Lavage Fluid Associated with EVALI. N Engl J Med. 2020 Feb 20;382(8):697-705. [CrossRef] [PubMed]
- Taylor J, Wiens T, Peterson J, et al. Characteristics of E-cigarette, or Vaping, Products Used by Patients with 18 and 2019. MMWR Morb Mortal Wkly Rep. 2019 Nov 29;68(47):1096-1100. [CrossRef] [PubMed]
- Rachid M, Yin J, Mier N, et al. Reversed Takutsubo Cardiomyopathy in a young patient with vaping induced acute lung injury. ATS International Conference Abstract Presentation. 2020 May 15-20. Philadelphia, PA. Abstract nr A1839.
- Aberegg SK, Maddock SD, Blagev DP, Callahan SJ. Diagnosis of EVALI: General Approach and the Role of Bronchoscopy. Chest. 2020 Aug;158(2):820-827. [CrossRef] [PubMed]
- Blagev DP, Harris D, Dunn AC, Guidry DW, Grissom CK, Lanspa MJ. Clinical presentation, treatment, and short-term outcomes of lung injury associated with e-cigarettes or vaping: a prospective observational cohort study. Lancet. 2019 Dec 7;394(10214):2073-2083. [CrossRef] [PubMed]
- Kalininskiy A, Bach CT, Nacca NE, et al. E-cigarette, or vaping, product use associated lung injury (EVALI): case series and diagnostic approach. Lancet Respir Med. 2019 Dec;7(12):1017-1026. [CrossRef] [PubMed]
- Heinzerling A, Armatas C, Karmarkar E, et al. Severe Lung Injury Associated With Use of e-Cigarette, or Vaping, Products-California, 2019. JAMA Intern Med. 2020 Jun 1;180(6):861-869. [CrossRef] [PubMed]
- Puebla Neira D, Tambra S, Bhasin V, Nawgiri R, Duarte AG. Discordant bilateral bronchoalveolar lavage findings in a patient with acute eosinophilic pneumonia associated with counterfeit tetrahydrocannabinol oil vaping. Respir Med Case Rep. 2020 Feb 3;29:101015. [CrossRef] [PubMed]
- Henry TS, Kanne JP, Kligerman SJ. Imaging of Vaping-Associated Lung Disease. N Engl J Med. 2019 Oct 10;381(15):1486-1487. [CrossRef] [PubMed]
- Thakrar PD, Boyd KP, Swanson CP, Wideburg E, Kumbhar SS. E-cigarette, or vaping, product use-associated lung injury in adolescents: a review of imaging features. Pediatr Radiol. 2020 Mar;50(3):338-344. [CrossRef] [PubMed]
- Larsen BT, Butt YM, Smith ML. More on the Pathology of Vaping-Associated Lung Injury. Reply. N Engl J Med. 2020 Jan 23;382(4):388-390. [CrossRef] [PubMed]
- Larcombe AN, Janka MA, Mullins BJ, Berry LJ, Bredin A, Franklin PJ. The effects of electronic cigarette aerosol exposure on inflammation and lung function in mice. Am J Physiol Lung Cell Mol Physiol. 2017 Jul 1;313(1):L67-L79. [CrossRef] [PubMed]
- Meo SA, Ansary MA, Barayan FR, Almusallam AS, Almehaid AM, Alarifi NS, Alsohaibani TA, Zia I. Electronic Cigarettes: Impact on Lung Function and Fractional Exhaled Nitric Oxide Among Healthy Adults. Am J Mens Health. 2019 Jan-Feb;13(1):1557988318806073. [CrossRef] [PubMed]
- Kotoulas SC, Pataka A, Domvri K, et al. Acute effects of e-cigarette vaping on pulmonary function and airway inflammation in healthy individuals and in patients with asthma. Respirology. 2020 Oct;25(10):1037-1045. [CrossRef] [PubMed]
- Choe J, Chen P, Falk JA, Nguyen L, Ng D, Parimon T, Ghandehari S. A Case Series of Vaping-Associated Lung Injury Requiring Mechanical Ventilation. Crit Care Explor. 2020 Jan 29;2(1):e0079. [CrossRef] [PubMed]
- Cerveri I, Corsico AG, Accordini S, et al. Underestimation of airflow obstruction among young adults using FEV1/FVC <70% as a fixed cut-off: a longitudinal evaluation of clinical and functional outcomes. Thorax. 2008 Dec;63(12):1040-5. [CrossRef] [PubMed]
Cite as: Espinosa RF, Hussein A, Sehring M, Rachid M, Dunn R, Taneja D. A Case Series of Electronic or Vaping Induced Lung Injury. Southwest J Pulm Crit Care. 2021;23(2):62-9. doi: https://doi.org/10.13175/swjpcc032-21 PDF