Evan Denis Schmitz, MD
Abstract
A patient receiving mechanical ventilation with multiple left hydropneumothoraces had a persistent air leak through the thoracostomy tube. The leak was temporarily resolved by interventional bronchoscopy at the bedside in the ICU. Because of the limited resources available at the hospital, a Swan-Ganz catheter was inserted into the left upper lobe bronchus, inflated and left in place. The air leak ceased and the left upper lobe bronchus was occluded with an autologous blood plug by infusing the patient’s own blood through the distal port of the catheter. The patient’s oxygenation improved significantly. The effects persisted for 2.5 hours until the air leak returned while the patient remained intubated. Such a technique may be useful when managing persistent air leaks.
Introduction
An air leak during mechanical ventilation despite the insertion of a thoracostomy tube can be detected by the bubbling of air through the air seal in the chest drainage system (1). A persistent air leak (PAL) is often defined as persistence of the air leak beyond 24 hours, which can hinder ventilation and inhibit lung expansion. Furthermore, the leak may inhibit healing of the fistula between the lung and the pleural space. Recommendations for the management of PALs include surgical repair as the gold standard for treatment (1,2). However, published anecdotal reports describe successful treatment of PALs with endobronchial insertion of fibrin sealants, ethanol injection, metal coils, Watanabe spigots and endobronchial valves. Success is also reported with chemical and autologous blood patch pleurodesis (1). We report a bedside interventional bronchoscopy technique using a Swan-Ganz catheter for the treatment of PALs while intubated and ventilated. A Swan-Ganz catheter is inserted into a lobar bronchus using direct visualization with a bronchoscope, the balloon is inflated and left in place while an autologous blood plug is created utilizing the distal port of the catheter.
Case Presentation
A 69-year-old man with no prior medical contact presented to the emergency department with severe shortness of breath and altered mental status. He was intubated in the emergency department for hypoxia. On arrival to the ICU he was in hypoxic respiratory failure and septic shock with a PaO2 in the 40s. His ventilator plateau pressures were 40-50 cm H2O. Chest radiography revealed moderate pneumomediastinum and multiple loculated hydropneumothoraces involving the left lung with suspected necrotic left upper lobe (Figure 1).
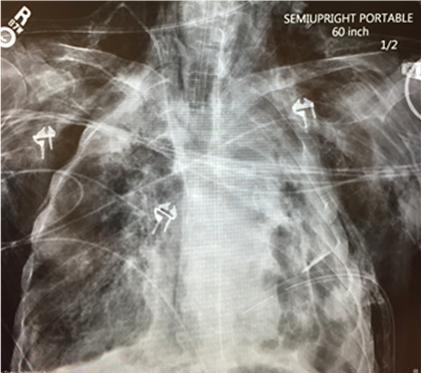
Figure 1. Portable AP chest x-ray showing left lung loculated hydropneumothoraces in the apex, medial and lateral walls of the left chest, subcutaneous emphysema, mediastinal emphysema and very low lung volumes. There are right apical and lower lobe areas of consolidation. A left thoracostomy tube is in place.
A 32 Fr thoracostomy tube was placed in the left intercostal space lateral to the nipple in the mid-axillary. The larger thoracostomy tube was chosen because of concern that the smaller pig-tailed catheters might not be adequate to control the leak. Plateau pressure improved to 30 cm H2O.
Despite a low tidal volume ventilator strategy and -40 cm H2O suction through the thoracostomy tube, the patient had an air leak through the thoracostomy tube which continued to bubble in the water seal chamber during both inspiration and expiration. The air leak did not improve over the ensuing 24 hours and subcutaneous emphysema worsened when attempts were made to decrease suction which was confirmed by physical exam and chest x-ray. Selective right lung ventilation led to inadequate ventilation as evidenced by increasing end-tidal CO2.
To determine and attempt to control the source of the persistent air leak, an interventional bronchoscopy was performed at bedside. Because other devices to such as metal coils, endobronchial valves, fibrin glue and a YAG laser were unavailable, a 6 Fr Swan-Ganz catheter was used. The Swan-Ganz catheter was threaded through the opening of the bronchoscope adaptor down the endotracheal tube to 3 cm above the carina. A flexible bronchoscope was then advanced along the side of the catheter through the bronchoscope adaptor and down the endotracheal tube. The catheter was not inside the working channel of the bronchoscope. The catheter was manipulated along with the bronchoscope, taking advantage of the inherent bend in the catheter, into the left mainstem bronchus and into the left upper lobe bronchus just distal to the lingular bronchus and inflated (Figure 2).
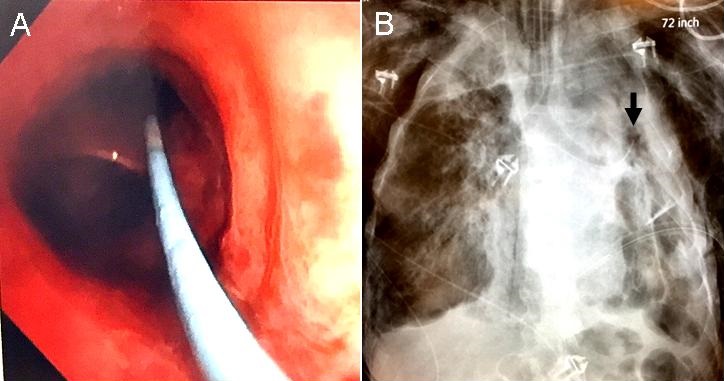
Figure 2. Panel A: Bronchoscopic view showing the Swan-Ganz catheter in the left upper lobe bronchus. Panel B: Chest x-ray confirming the Swan-Ganz catheter in the left upper lobe with the balloon inflated (arrow).
The massive air leak stopped completely. A blood plug was then created by instilling 20 ml of the patient’s own blood into the distal port of the catheter distal to the balloon along with 5 ml of 1:1000 epinephrine. The bronchoscope was used to hold the balloon in place for 10 minutes while the blood clotted. The bronchoscope was carefully removed and the catheter with the balloon inflated was left in place (Figure 3).
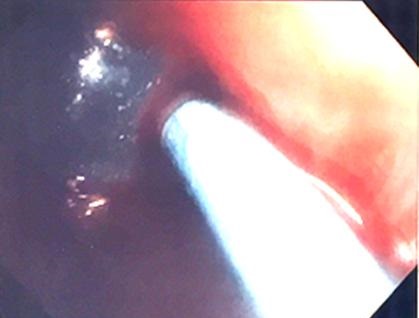
Figure 3. Bronchoscopic view showing the catheter passing into the left upper lobe bronchus with the surrounding blood plug.
The bronchoscope adaptor was taped post-bronchoscopy at the opening with an occlusive dressing so no air could leak around the catheter. The patient tolerated the procedure well. The air leak was successfully stopped with no evidence of worsening pneumothoraces. After PaO2 increased from the 40s on admission to the 170s after the PAL was stopped. Chest x-ray at 1 and 3 hours showed no evidence of worsening pneumothorax with the Swan-Ganz catheter still in place and inflated in the left upper lobe bronchus. After 2.5 hours, a smaller air leak did return but was present only during inspiration.
Discussion
A PAL during mechanical ventilation can be a serious complication of ventilator therapy. It can lead to poor lung expansion, ventilation/perfusion mismatch, direct extension of airway infection into the pleural space, and an inability to maintain positive end-expiratory pressure. Patients with a PAL have increased complications, including ICU readmission, pneumonia, and a longer hospital stay (3,4). Fortunately, it appears to be relatively rare. In a retrospective study only 39 out of 1,700 mechanically ventilated patients had a PAL defined as lasting for greater than 24 hours (5).
The American College of Chest Physicians guidelines published in 2001 and the 2010 British Thoracic Society guidelines on pleural disease recommend waiting for about 4 days and then seeking surgical evaluation for a PAL (2,6). It was recommended that consideration should be given to placing the thoracostomy tube to water seal rather than to suction. However, this may not be possible in patients with a large persistent air leak that complicates ventilation. In those instances, a variety of endobronchial and pleural interventions have been attempted. Although the reports are anecdotal, most achieved success with either none or minimal complications (1). There have been two basic approaches to treat PALs; sealing the air leak from the bronchial side or from the pleural side. Those therapies administered through the bronchoscope include fibrin sealant, metal coils, Watanabe spigots, synthetic hydrogel, platelet gel, endobronchial valves and YAG laser (1). Complications were infrequent and minor. Ethanolamine and ethanol have also been used but there appear to be more complications with those treatments. From the pleural side, blood patch and chemical pleurodesis have been used successfully (1). However, chemical pleurodesis might result in a trapped lung.
The technique reported here can be performed with materials available in the ICU. A torqueable guidewire can be inserted if needed to help increase the catheter stiffness and help with advancement of the catheter into the individual bronchus to identify the source of the bronchopleural fistula. Alternatives to a blood patch might include occlusion of the culprit bronchus with the patient’s own mucus and argon plasma coagulation to form a clot. A blood patch can be used to determine the potential success of a more permanent material to occlude the bronchus, such as a fibrin seal, synthetic hydrogel, laser, or before attempting endobronchial valve placement.
Conclusion
Bedside endobronchial management of PAL is feasible using a flexible bronchoscope and Swan-Ganz catheter for localization, tamponade and delivery of a blood plug.
References
-
Dugan KC, Laxmanan B, Murgu S, Hogarth DK. Management of persistent air leaks. Chest. 2017;152(2):417-23. [CrossRef] [PubMed]
-
Baumann MH, Strange C, Heffner JE. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest. 2001;119(2):590-602. [CrossRef] [PubMed]
-
Liberman M, Muzikansky A, Wright CD, et al. Incidence and risk factors of persistent air leak after major pulmonary resection and use of chemical pleurodesis. Ann Thorac Surg. 2010;89(3):891-897. [CrossRef] [PubMed]
-
DeCamp MM, Blackstone EH, Naunheim KS, et al. Patient and surgical factors influencing air leak after lung volume reduction surgery: lessons learned from the National Emphysema Treatment Trial. Ann Thorac Surg. 2006;82(1):197-206. [CrossRef] [PubMed]
-
Pierson DJ, Horton CA, Bates PW. Persistent bronchopleural air leak during mechanical ventilation. A review of 39 cases. Chest. 1986;90(3):321-3. [CrossRef] [PubMed]
-
Havelock T, Teoh R, Laws D, et al. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65(suppl 2):ii61-ii76. [CrossRef] [PubMed]
Cite as: Schmitz ED. A new interventional bronchoscopy technique for the treatment of bronchopleural fistula. Southwest J Pulm Crit Care. 2017;15(4):174-8. doi: https://doi.org/10.13175/swjpcc120-17 PDF